Top Qs
Timeline
Chat
Perspective
Molybdenum tetrachloride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Molybdenum tetrachloride is the inorganic compound with the empirical formula MoCl4. The material exists as two polymorphs, both being dark-colored paramagnetic solids. These compounds are mainly of interest as precursors to other molybdenum complexes.
Remove ads
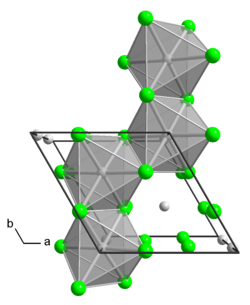
Structure

The α polymorph is a polymer. The β polymorph is a hexamer. In both polymorph, the Mo center is octahedral with two terminal chloride ligands and four doubly bridging ligands.[1] In addition to these two binary phases, a number of adducts are known with the formula MoCl4L2 where L is a Lewis base.
Preparation
α-Molybdenum tetrachloride can be prepared from by dechlorination of molybdenum pentachloride using tetrachloroethene:[2]
- 2 MoCl5 + C2Cl4 → 2 MoCl4 + C2Cl6
Heating α-molybdenum tetrachloride in a sealed container in the presence of molybdenum pentachloride induces conversion to the β polymorph.[2]
Reactions
When heated in an open container, molybdenum tetrachloride evolves chlorine, giving molybdenum trichloride;[2]
- 2 MoCl4 → 2 MoCl3 + Cl2
The acetonitrile complex adduct can be prepared by reduction of the pentachloride with acetonitrile:[3][4]
- 2 MoCl5 + 5 CH3CN → 2 MoCl4(CH3CN)2 + ClCH2CN + HCl
The MeCN ligands can be exchanged with other ligands:
- MoCl4(CH3CN)2 + 2 THF → MoCl4(THF)2 + 2 CH3CN
The pentachloride can be reduced to the ether complex MoCl4(Et2O)2 using tin powder. It is a beige, paramagnetic solid.[5]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads