Top Qs
Timeline
Chat
Perspective
Molybdenum(III) chloride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Molybdenum(III) chloride is the inorganic compound with the formula MoCl3. It forms purple crystals.[1]
Remove ads
Synthesis and structure
Molybdenum(III) chloride is synthesized by the reduction of molybdenum(V) chloride with hydrogen.[2] A higher yield is produced by the reduction of pure molybdenum(V) chloride with anhydrous tin(II) chloride as the reducing agent.[3]
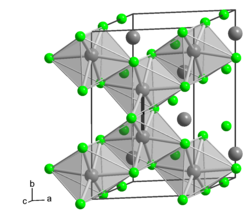
Molybdenum trichloride exists as two polymorphs: alpha (α) and beta (β). The alpha structure is similar to that of aluminum chloride (AlCl3). In this structure, molybdenum has octahedral coordination geometry and exhibits cubic close-packing in its crystalline structure. The beta structure, however, exhibits hexagonal close packing.[4]
Remove ads
Ether complexes
Molybdenum trichloride gives a ether complexes MoCl3(thf)3 and MoCl3(Et2O)3. They are beige, paramagnetic solids. Both feature octahedral Mo centers. The diethyl ether complex is synthesized by reducing a Et2O solution of MoCl5 with tin powder.[5] Older procedures involve stepwise reduction involving isolation of the Mo(IV)-thf complex.[6]
Hexa(tert-butoxy)dimolybdenum(III) is prepared by the salt metathesis reaction from MoCl3(thf)3:[7]
- 2 MoCl3(thf)3 + 6 LiOBu-t → Mo2(OBu-t)6 + 6 LiCl + 6 thf
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads