Top Qs
Timeline
Chat
Perspective
Molybdenum(V) chloride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Molybdenum(V) chloride is the inorganic compound with the empirical formula MoCl5. This dark volatile solid is used in research to prepare other molybdenum compounds. It is moisture-sensitive and soluble in chlorinated solvents.
Remove ads
Structure

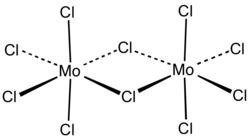
Usually called molybdenum pentachloride, it is in fact partly a dimer with the molecular formula Mo2Cl10.[1] In the dimer, each molybdenum has local octahedral symmetry and two chlorides bridge between the molybdenum centers.[2] A similar structure is also found for the pentachlorides of W, Nb and Ta.[3] In the gas phase and partly in solution, the dimers partially dissociate to give a monomeric MoCl5.[4] The monomer is paramagnetic, with one unpaired electron per Mo center, reflecting the fact that the formal oxidation state is +5, leaving one valence electron on the metal center.
Remove ads
Preparation and properties
MoCl5 is prepared by chlorination of Mo metal but also chlorination of MoO3. The unstable hexachloride MoCl6 is not produced in this way.[5]
MoCl5 is reduced by acetonitrile:[6]
- 2 MoCl5 + 5 CH3CN → 2 MoCl4(CH3CN)2 + HCl + ClCH2CN
Molybdenum(IV) bromide is prepared by treatment of MoCl5 with hydrogen bromide:
- 2 MoCl5 + 10 HBr → 2 MoBr4 + 10 HCl + Br2
The reaction proceeds via the unstable molybdenum(V) bromide, which releases bromine at room temperature.[7]
MoCl5 is a good Lewis acid toward non-oxidizable ligands. It forms an adduct with chloride to form [MoCl6]−. In organic synthesis, the compound finds occasional use in chlorinations, deoxygenation, and oxidative coupling reactions.[8]
Although it polymerizes tetrahydrofuran, MoCl5 is stable in diethyl ether. Reduction of such solutions with tin gives MoCl4((CH3CH2)2O)2 and MoCl3((CH3CH2)2O)3, depending on conditions.[9]
Remove ads
Safety considerations
MoCl5 is an aggressive oxidant and readily hydrolyzes to release HCl.
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads