Top Qs
Timeline
Chat
Perspective
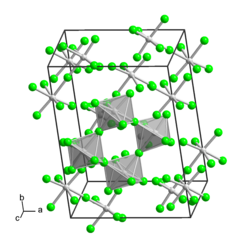
Molybdenum(V) fluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Molybdenum(V) fluoride is an inorganic compound with the formula MoF5. It is a hygroscopic yellow solid. Like most pentafluorides, it exists as a tetramer.[2]
Remove ads
Production
Molybdenum(V) fluoride is produced by the reaction of molybdenum and molybdenum hexafluoride:[3]
- Mo + 5 MoF6 → 6 MoF5
It can also be prepared by the reduction of molybdenum hexafluoride with phosphorus trifluoride or tungsten hexacarbonyl, or by the oxidation of elemental molybdenum with fluorine at 900 °C.[3]
About 165 °C, it disproportionates to the tetra- and hexafluoride:[1]
- 2 MoF5 → MoF4 + MoF6
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads