Top Qs
Timeline
Chat
Perspective
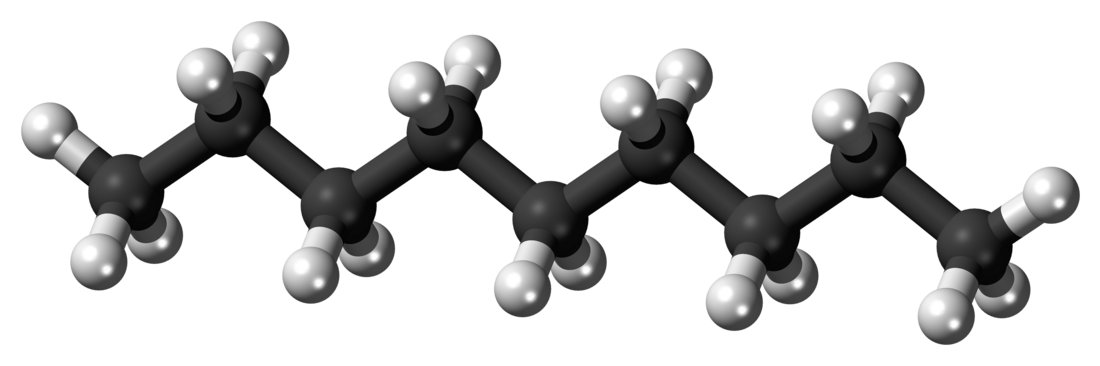
Nonane
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Nonane is a linear alkane hydrocarbon with the chemical formula C9H20. It is a colorless, flammable liquid, occurring primarily in the component of the petroleum distillate fraction commonly called kerosene, which is used as a heating, tractor, and jet fuel.[4] Nonane is also used as a solvent, distillation chaser, fuel additive, and a component in biodegradable detergents.[5] It is also a minor component of diesel fuel.[6]
Nonane has 35 structural isomers.
Its substituent form is nonyl. Its cycloalkane counterpart is cyclononane, (C9H18).
Unlike most alkanes, the numeric prefix in its name is from Latin, not Greek. (A name using a Greek prefix would be enneane.)
Remove ads
Combustion reactions
Nonane undergoes combustion reactions that are similar to other alkanes. In the presence of sufficient oxygen, nonane burns to form water and carbon dioxide.
When insufficient oxygen is available for complete combustion, the burning products include carbon monoxide.
- 2C9H20 + 19O2 → 18CO + 20H2O
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads