Top Qs
Timeline
Chat
Perspective
Platinum(IV) iodide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Platinum(IV) iodide is a inorganic compound with the formula PtI4.[1] it is a dark brown diamagnetic solid and is one of several binary iodides of platinum.
Remove ads
Preparation
Platinum(IV) iodide can be prepared from the effect of iodine on platinum:[2]
- Pt + 2I2 → PtI4
Iodide accelerates this process.[3]
It can also be obtained from the decomposition of hydrogen hexaiodoplatinate(IV) at 80 °C:
- H2[PtI6] → PtI4 + 2HI
Physical properties
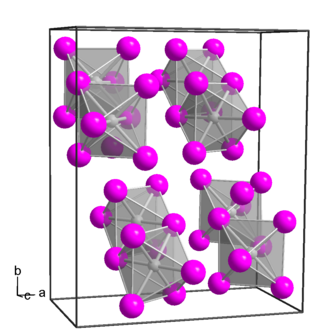
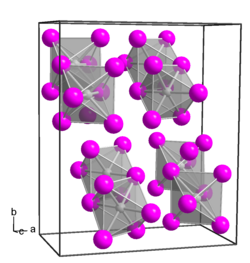
Platinum(IV) iodide forms dark brown crystals of several modifications:[4]
- α-PtI4, rhombic crystal system, spatial group P bca,[5] cell parameters a = 1.290 nm, b = 1.564 nm, c = 0.690 nm, Z = 8;
- β-PtI4, cubic crystal system, spatial group P m3m, cell parameters a = 0.56 nm, Z = 1;
- γ-PtI4, tetragonal crystal system, spatial group I 41/a, cell parameters a = 0.677 nm, c = 3.110 nm, Z = 8.
Platinum(IV) iodide decomposes in water. It is also soluble in ethanol, acetone, alkali, HI, KI, liquid NH3.[6]
Remove ads
Chemical properties
It decomposes when heated:
- PtI4 → Pt + 2I2
When dissolved in hydroiodic acid, platinum(IV) iodide forms hydrogen hexaiodoplatinate(IV):
- PtI4 + 2HI → H2[PtI6]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads