Top Qs
Timeline
Chat
Perspective
Potassium fluoride
Ionic compound (KF) From Wikipedia, the free encyclopedia
Remove ads
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide salt and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective.
Remove ads
Preparation
Potassium fluoride is prepared by reacting potassium carbonate with hydrofluoric acid. Evaporation of the solution forms crystals of potassium bifluoride. The bifluoride on heating yields potassium fluoride:
- K2CO3 + 4 HF → 2 KHF2 + CO2 ↑ + H2O
- KHF2 → KF + HF ↑
Platinum or heat resistant plastic containers are often used for these operations.
Potassium chloride converts to KF upon treatment with hydrogen fluoride. In this way, potassium fluoride is recyclable.[3]
Remove ads
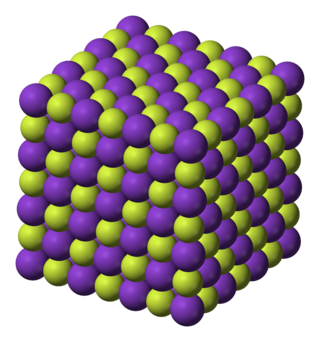
Crystalline properties
KF crystallizes in the cubic NaCl crystal structure. The lattice parameter at room temperature is 0.266 nm.[4]
Applications in organic chemistry
In organic chemistry, KF can be used for the conversion of chlorocarbons into fluorocarbons, via the Finkelstein (alkyl halides)[5] and Halex reactions (aryl chlorides).[3] Such reactions usually employ polar solvents such as dimethyl formamide, ethylene glycol, and dimethyl sulfoxide.[6] More efficient fluorination of aliphatic halides can be achieved with a combination of crown ether and bulky diols in acetonitrile solvent.[7]
Potassium fluoride on alumina (KF/Al2O3) is a base used in organic synthesis. It was originally introduced in 1979 by Ando et al. for inducing alkylation reactions.[8]
Safety considerations
Like other sources of the fluoride ion, F−, KF is poisonous, although lethal doses approach gram levels for humans. It is harmful by inhalation and ingestion. It is highly corrosive, and skin contact may cause severe burns.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads