Top Qs
Timeline
Chat
Perspective
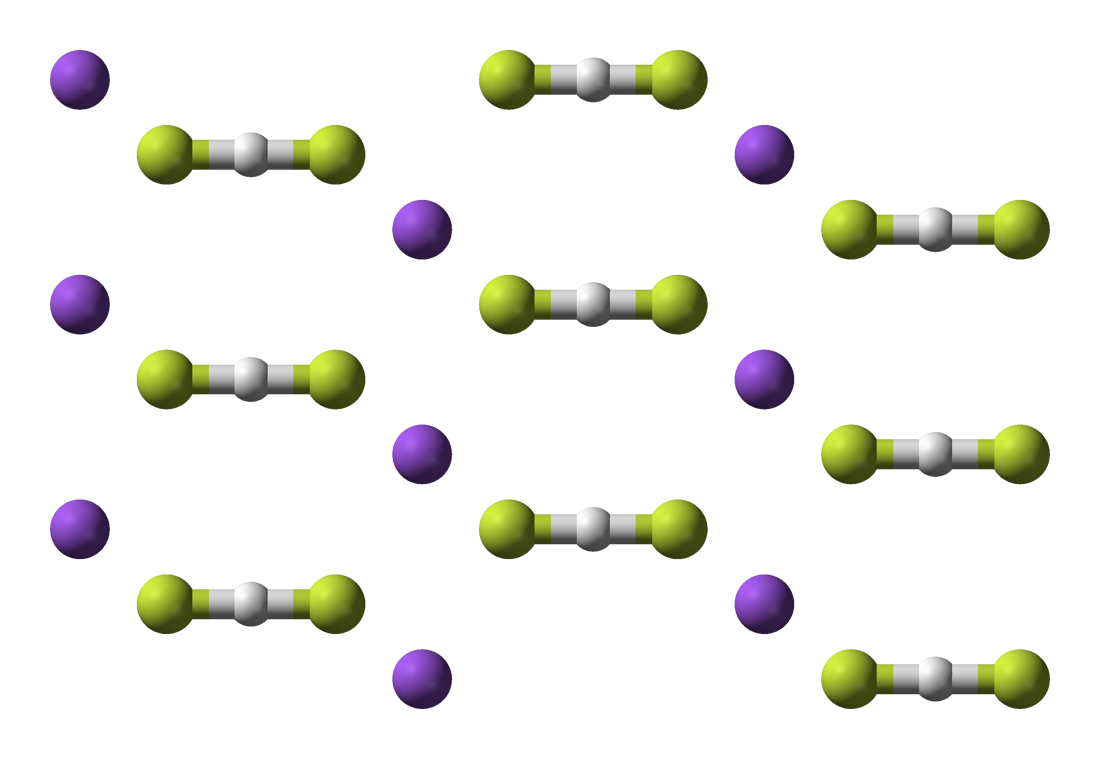
Potassium bifluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Potassium bifluoride is the inorganic compound with the formula K[HF2]. This colourless salt consists of the potassium cation (K+) and the bifluoride anion ([HF2]−). The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commercial use as an etchant as well as in cleaning products.[3]
Remove ads
Synthesis and reactions
The salt was prepared by Edmond Frémy by treating potassium carbonate or potassium hydroxide with hydrofluoric acid:
- 2 HF + KOH → K[HF2] + H2O
With one more equivalent of HF, K[H2F3] (CAS RN 12178-06-2, m.p. 71.7 °C[4]) is produced:
- HF + K[HF2] → K[H2F3]
Thermal decomposition of K[HF2] gives hydrogen fluoride:
- K[HF2] → HF + KF
Remove ads
Applications
The industrial production of fluorine entails the electrolysis of molten K[HF2] and K[H2F3].[3] The electrolysis of K[HF2] was first used by Henri Moissan in 1886.
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads