Top Qs
Timeline
Chat
Perspective
Pyrazole
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
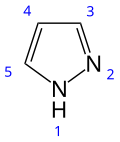
Pyrazole is an organic compound with the formula (CH)3N2H. It is a heterocycle characterized as an azole with a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Pyrazole itself has few applications but many substituted pyrazoles are of commercial interest. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.
Remove ads
Properties
Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C).[3] According to X-ray crystallography, the compound is planar. The two C-N distances are similar, both near 1.33 Å[4]
History
The term pyrazole was given to this class of compounds by German Chemist Ludwig Knorr in 1883.[5] In a classical method developed by German chemist Hans von Pechmann in 1898, pyrazole was synthesized from acetylene and diazomethane.[6]
Preparation
Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation:[7]
Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine (Knorr-type reactions).[8][9] For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole:[10]
- CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O
A wide variety of pyrazoles can be made so:[8]
Occurrence and uses
Summarize
Perspective

In 1959, the first natural pyrazole, 1-pyrazolyl-alanine, was isolated from seeds of watermelons.[11][12]
In medicine, derivatives of pyrazole are widely used,[13] including celecoxib and similar COX-2 inhibitors, zaleplon, betazole, and CDPPB.[14] The pyrazole ring is found within a variety of pesticides as fungicides, insecticides and herbicides,[13] including fluoxapiprolin,[15] fenpyroximate, fipronil, tebufenpyrad and tolfenpyrad.[16] Pyrazole moieties are listed among the highly used ring systems for small molecule drugs by the US FDA[17]
3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid is used in the manufacture of six commercial fungicides which are inhibitors of succinate dehydrogenase.[18][19]
Pyrazole is an inhibitor of the alcohol dehydrogenase enzyme, and, as such, is used as an adjuvant with ethanol, to induce alcohol dependency in experimental laboratory mice.[20]
Conversion to scorpionates
Pyrazoles react with potassium borohydride to form a class of ligands known as scorpionate. Pyrazole itself reacts with potassium borohydride at high temperatures (~200 °C) to form a tridentate ligand known as Tp ligand:
Remove ads
See also
- 3,5-dimethylpyrazole
- Pyrazolidine, fully saturated analogue
- imidazole, structural analogue of pyrazole with two non-adjacent nitrogen atoms.
- isoxazole, another analogue, the nitrogen atom in position 1 replaced by oxygen.
References
Further reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads