Top Qs
Timeline
Chat
Perspective
RNASEH2B
Protein-coding gene in the species Homo sapiens From Wikipedia, the free encyclopedia
Remove ads
Ribonuclease H2, subunit B is a protein in humans that is encoded by the RNASEH2B gene.[5] RNase H2 is composed of a single catalytic subunit (A) and two non-catalytic subunits (B and C), and it degrades the RNA of RNA:DNA hybrids. The non-catalytic B subunit of RNase H2 is thought to play a role in DNA replication and repair.[5]
Mutations in this gene are a cause of Aicardi-Goutieres syndrome type 2 (AGS2).[5][6]
Remove ads
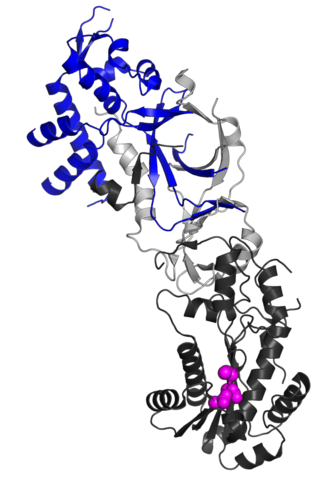
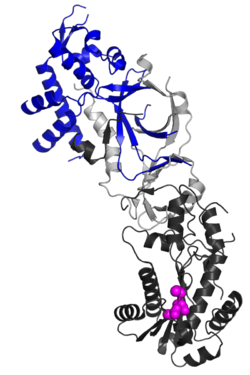
Structure
The RNASEH2B protein is a auxiliary subunit of the heterotrimeric RNase H2 complex, which includes catalytic subunit RNASEH2A and another accessory subunit, RNASEH2C.[7][8] Structurally, RNASEH2B forms a stable heterodimer with RNASEH2C, contributing to an interwoven triple β-barrel fold that integrates with the C-terminal extension of RNASEH2A.[7] This architecture stabilizes the complex and enables enzymatic activity. A key functional feature of RNASEH2B is its C-terminal PIP box (PCNA-interacting peptide) motif, which directly binds Proliferating Cell Nuclear Antigen (PCNA).[9] This interaction localizes RNase H2 to replication foci, ensuring its role in processing misincorporated ribonucleotides during DNA replication and repair.[9]
Remove ads
Function
The RNASEH2B protein functions as a non-catalytic auxiliary subunit of the RNase H2 enzyme complex, critical for maintaining genome stability during DNA replication and repair. It forms a heterotrimeric complex with RNASEH2A (catalytic subunit) and RNASEH2C, where RNASEH2B and RNASEH2C create a structural scaffold essential for stabilizing RNASEH2A's enzymatic activity.[10] A key feature of RNASEH2B is its C-terminal PIP-box motif, which directly binds Proliferating Cell Nuclear Antigen (PCNA), localizing the RNase H2 complex to DNA replication foci.[10] This interaction enables the enzyme to processively remove misincorporated ribonucleotides from DNA, a critical function given that replicative DNA polymerases erroneously incorporate ribonucleotides at rates of ~1 per 7,600 nucleotides.[10] Beyond its role in replication, RNASEH2B contributes to RNase H2's ability to cleave diverse RNA/DNA hybrids, suggesting broader involvement in resolving transcription-associated R-loops and maintaining genomic surveillance.[10]
Remove ads
Clinical significance
Mutations disrupting the interface between RNASEH2B, RNASEH2C, and RNASEH2A are linked to Aicardi-Goutières Syndrome, highlighting the structural importance of these subunits in maintaining complex stability and function.[7][8] Mutations in RNASEH2B disrupt the complex's structural integrity and PCNA binding capacity, leading to defective ribonucleotide excision repair and causing Aicardi-Goutières Syndrome, an autoinflammatory disorder linked to accumulated DNA damage.[10]
Mutagenesis studies
Summarize
Perspective
Knockout of the RNASEH2B gene in mice leads to early embryonic lethality. To investigate its function, genetically engineered mice with a premature stop codon in exon 7 of the Rnaseh2b gene were created.[11] It was hypothesized that the observed growth arrest in these mice results from a p53-dependent DNA damage response triggered by the accumulation of single ribonucleotides (RN) in genomic DNA.
Ribonucleotides accumulate in RNASEH2-null cells due to incorporation by replicative DNA polymerases. This incorporation occurs naturally in metazoans and leads to lesions—single ribonucleotides or diRN—covalently embedded in genomic DNA. These lesions occur at a frequency of approximately 1,000,000 sites per cell, making ribonucleotide incorporation the most common endogenous base lesion in the mammalian genome. The frequency aligns with predictions based on in vitro misincorporation rates by eukaryotic replicative polymerases.
The RNASEH2 complex serves as a genome surveillance enzyme essential for the removal of these ribonucleotides. The accumulation of ribonucleotides in the genomic DNA of RNASEH2-null mice implicates the RNASEH2 complex in maintaining genome integrity. These lesions are harmful, as the 2’-hydroxyl group of ribose increases the susceptibility of adjacent phosphodiester bonds to hydrolysis. In fact, it has been reported[who?] that ribonucleotides are incorporated at a rate of approximately one every 7,600 nucleotides in RNASEH2-null cells, amounting to around 1,300,000 lesions per cell. This estimate aligns with in vitro rates of misincorporation by eukaryotic replicative polymerases.
Misincorporated ribonucleotides can induce DNA damage. Importantly, ribonucleotides do not prevent DNA replication; polDNA can tolerate templates containing ribonucleotides, and early embryogenesis proceeds normally. The problem arises when excessive numbers of ribonucleotides are incorporated. DNA damage response signaling may be activated by ribonucleotides in difficult-to-replicate regions or near other lesions. Chromosomal rearrangements, including DNA breaks, have also been observed, possibly originating from replication fork collapse or hydrolysis of ribonucleotides on opposing DNA strands. The marked activation of DNA damage signaling in embryos may lead to a p53-mediated inhibition of proliferation, which likely contributes to the lethality observed in RNASEH2B-null embryos.
Remove ads
References
Further reading
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads