Top Qs
Timeline
Chat
Perspective
RNase MRP
RNA family From Wikipedia, the free encyclopedia
Remove ads
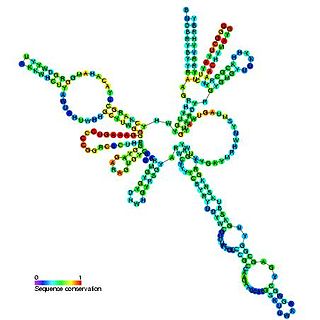
RNase MRP is an enzymatically active ribonucleoprotein with two distinct roles in eukaryotes. RNase MRP stands for RNase for Mitochondrial RNA Processing. In mitochondria, it plays a direct role in the initiation of mitochondrial DNA replication. In the nucleus, it is involved in precursor rRNA processing, where it cleaves the internal transcribed spacer 1 between 18S and 5.8S rRNAs.[1] Despite distinct functions, RNase MRP has been shown to be evolutionarily related to RNase P. Like eukaryotic RNase P, RNase MRP is not catalytically active without associated protein subunits.[2]
Mutations in the RNA component of RNase MRP cause cartilage–hair hypoplasia, a pleiotropic human disease. Responsible for this disease is a mutation in the RNase MRP RNA gene (RMRP), a non-coding RNA gene. RMRP was the first non-coding nuclear RNA gene found to cause disease.[3]
Remove ads
Components
Human RNase MRP form two sizes of ribonucleoproteins, 12S and 60–80S. The latter do not have RPP25 and RPP20. It appears to lack many protein subunits found in human RNase P.[4]
The yeast RNase MRP is more similar to its RNase P in that it also binds Pop4 (Rpp29), Pop6, Pop7 (Rpp20), Pop8 in addition to these proteins. It also binds Snm1, which is homologous to Rpr2 (Rpp21) in its RNaseP. The protein Rmp1 is unique to the yeast RNase MRP.[6]
Remove ads
Function
Summarize
Perspective
rRNA processing

RNase MRP and its role in pre-rRNA processing has been previously studied in Yeast cells. RNase MRP has been shown to cleave an internal transcribed spacer, specifically ITS1 at the specific site A3 of the rRNA precursor, leading, after additional trimming, to the formation of the mature 5′-end of 5.8S rRNA. Recent data that has been gathered using several temperature-sensitive RNase MRP mutants that showed that inactivation of RNase MRP leading to severe reduction of the abundance of all early intermediates in the typical rRNA processing pathway. However, the transcription of the rRNA precursor is not affected, thus suggesting that RNase MRP plays a key role in the processing of rRNA beyond the cleavage of the A3 site in ITS1.
Cell cycle via CLB2 5′-UTR clevage

Further research in Yeast cell RNase MRP has shown a potential role in the regulation of the cell cycle. RNase MRP mutations led to missegregation of plasmids and caused cell cycle delay at the end of mitosis, followed by a buildup of cyclin B2 (CLB2) protein (resulting from increased CLB2 mRNA concentration that codes for the CLB2 protein). RNase MRP also demonstrated cleavage ability of the 5′-UTR of CLB2 mRNA that allows for rapid 5′-to-3′ degradation by XRN1, an exoribonuclease enzyme.[7]
Remove ads
Link to RNAse P
Summarize
Perspective

RNase P and RNAse MRP are ribonucleoprotein complexes acting as ribonucleases in RNA processing. The RNA parts of both share a common arrangement of secondary structure in the catalytic (C) domain while their specificity (S) domains differ noticeably.[6] The two C domains share many conserved regions: sequences of the CR-I, CR-V, and CR-IV genes in domain 1 of the P4 helical region are conserved, with the consensus sequence in CR-IV being AGNNNNA for RNAse P and AGNNA for RNase MRP. CR-II and CR-III are also conserved in domain 2 of P RNA. The P3 helix is also conserved in both ribonucleases in all eukaryotes, but the function of this helix is not yet clear.[8]
The similarity in their RNA secondary structure, RNA sequences, and protein partners show that they most likely share a common ancestor. RNase MRP is an eukaryotic innovation, performing a function not seen in any RNase P, which is the conversion of preribosomal RNA into mature rRNA through splicing, modifications, and cleavage. The exact mechanism is described above.[8]
The broder RNase P and RNase MNP both share a modular arrangement, which can be broken into a "lid", a "base", and a "rear".[9] Most of the difference between the two are, as expected, involved in contacting the RNA substrate.[6]
Diseases associated with RNAse MRP gene
Summarize
Perspective
Metaphyseal dysplasia without hypotrichosis (MDWH), anauxetic dysplasia (AD), kyphomelic dysplasia (KD), Omenn syndrome (OS) are diseases associated with mutated and (or) dysfunctional RNAse MRP activity, hence, the RMRP gene.
Cartilage–hair hypoplasia
Mutations in the RNA component of RNase MRP cause cartilage–hair hypoplasia(CHH), a pleiotropic human disease. Two categories of mutations involving RNAse MRP have been identified in patients with CHH. The first type is when an insertion, duplication, or triplication occurs at the promoter of the RNAse MRP gene between the TATA box and the transcription initiation site. This causes the initiation of RNAse MRP to be slow, or to not occur at all. The second category consists of mutations that are in the transcribed RNA made by the RNAse MRP. Patients with CHH have been identified to have over 70 different mutations in the RNA transcript made by RNAse MRP, whereas around 30 distinct mutations have been identified in the promoter region of the RNAse MRP gene. Most CHH patients have a combination of either a promoter mutation in one allele along with a RNAse MRP RNA mutation in the other allele, or a combination of two RNAse MRP RNA mutations in both alleles. The fact that there is not often a mutation in the promoter region in both alleles shows the lethality of not having this RNA present that is transcribed by RNAse MRP.[10][11][12]
Metaphyseal dysplasia without hypotrichosis
Metaphyseal dysplasia Without Hypotrichosis (MDWH) patients are unable to produce normal, new tubular structures in the metaphyses of long bones. People diagnosed with MDWH will therefore tend to experience porous and expanded long bones. The mutation occurs on the RMRP gene in MDWH; the common insertion being (-21-20 insTCTGTGAAGCTGGGGAC) on the paternal allele and a 218A→G point mutation occurring on the maternal allele. MDWH is most likely a variant of CHH. They are the same in that they both display short stature. Some of the same genes involved in the mutations in CHH are the same genes that are mutated in MDWH.[13] These two diseases do differ in that MDWH lacks immunodeficiency and other skeletal features found in CHH patients.[3]
Anauxetic dysplasia
AD is an autosomal recessive spondylometaepiphyseal dysplasia typically characterized by an early (prenatal) onset of extremely short stature and adults that do not typically exceed 85 cm in height. A less than normal amount of teeth and slight mental retardation are also typical of AD. The associated mutation(s) are a homozygous insertion mutation and two compound heterozygous mutations.[3] Mutations in the promoter 5' regulatory region have been associated with this severe skeletal disease. Other names used to describe this condition are spondylometaepiphyseal dysplasia, anauxetic type, spondylometaepiphyseal dysplasia, Menger type.[14]
Kyphomelic dysplasia
KD is a form of short-limbed dwarfism. Characteristics of KD are bowing of long bones, dysmorphia, flattened vertebrae, and short ribs. Femoral bowing is the hallmark diagnostic characteristic of KD. Novel mutations have been discovered in the RMRP gene of a single patient with KD, specifically, a mutation (insertion) of T at 194-195 paternal allele and a 63C-->T point mutation of the maternal allele. As with OS, the MSRP gene has not been strictly linked to the diseases but current research is suggestive that the MSRP gene is a factor. KD has been observed in very few patients yet this sublethal disease remains relevant to discussions of the distinct manifestations of minimal change disease. KD is rather similar to several forms of MCD in that it exhibits combined immune deficiency and aplastic anemia.[3]
Omenn syndrome
Omenn syndrome (OS) is a severe immunodeficiency disease, mostly characterized by scaly erythroderma and severe reddening of the skin. OS is also commonly accompanied by enlarged lymphoid tissues, protracted diarrhea, failure to thrive, and eosinophilia. Gene sequences of people with OS reveal three novel mutations in the RMRP gene, suggesting a link to the RMRP gene, but research is ongoing to better ascertain the cause of OS. At the moment there exists only one treatment for OS which is bone marrow transplantation. If no treatment is performed OS is rather fatal resulting in death in infancy. Patients with OS are immunodeficient meaning their immune system is compromised and cannot properly fight infections resulting in serious secondary illnesses.[3]
Remove ads
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads