Top Qs
Timeline
Chat
Perspective
Tellurium tetraiodide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
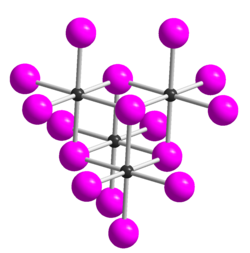
Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4.[2] In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.[2]
Remove ads
Preparation
Tellurium tetraiodide can be prepared by reacting Te and iodomethane, CH3I.[2] In the vapour TeI4 dissociates:[3]
- TeI4 → TeI2 + I2
It can be also obtained by reacting telluric acid with hydrogen iodide.[4]
- Te(OH)6 + HI → TeI4 + I2 + 6 H2O
It can also be obtained by reacting the elements, which can also produce tellurium diiodide and tellurium monoiodide, depending on the reaction conditions:[5]
- Te + 2 I2 → TeI4
- TeI4 → TeI2 + I2
Remove ads
Properties
Tellurium tetraiodide is an iron-gray solid that decomposes slowly in cold water and quickly in warm water to form tellurium dioxide and hydrogen iodide.[6] It is stable even in moist air and decomposes when heated, releasing iodine. It is soluble in hydriodic acid to form H[TeI5] and it is slightly soluble in acetone.[4]
Tellurium tetraiodide is a conductor when molten, dissociating into the ions TeI3+ and I−. In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:[3]
- TeI4 + 2 CH3CN → (CH3CN)2TeI3+ + I−
Five modifications of tellurium tetraiodide are known, all of which are composed of tetrameric molecules.[7] The δ form is the most thermodynamically stable form. This is structurally derived (as well as the α, β and γ forms) from the ε form.
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads