Top Qs
Timeline
Chat
Perspective
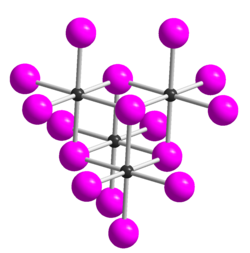
Tellurium tetrabromide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Tellurium tetrabromide (TeBr4) is an inorganic chemical compound. It has a similar tetrameric structure to TeCl4.[3] It can be made by reacting bromine and tellurium.[4] In the vapour TeBr4 dissociates:[3]
- TeBr4 → TeBr2 + Br2
It is a conductor when molten, dissociating into the ions TeBr3+ and Br−. When dissolved in benzene and toluene, TeBr4 is present as the unionized tetramer Te4Br16.[3] In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:
- TeBr4 + 2CH3CN → (CH3CN)2TeBr3+ + Br−
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads