Top Qs
Timeline
Chat
Perspective
Tert-Butyl chloride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
tert-Butyl chloride is the organochloride with the formula (CH3)3CCl. It is a colorless, flammable liquid. It is sparingly soluble in water, with a tendency to undergo hydrolysis to the corresponding tert-butyl alcohol. It is produced industrially as a precursor to other organic compounds.[1]
Remove ads
Synthesis
tert-Butyl chloride is produced by the reaction of tert-butyl alcohol with hydrogen chloride.[1] In the laboratory, concentrated hydrochloric acid is used. The conversion entails a SN1 reaction as shown below.[2]
| Step 1 | Step 2 | Step 3 |
 | 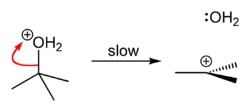 |  |
| The acid protonates the alcohol, forming a good leaving group (water). | Water leaves the protonated t-BuOH, forming a relatively stable tertiary carbocation. | The chloride ion attacks the carbocation, forming t-BuCl. |
The overall reaction, therefore, is:
- (CH3)3COH + HCl → (CH3)3CCl + H2O
Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the step 2 allows the SN1 mechanism to be followed, whereas a primary alcohol would follow an SN2 mechanism.
Remove ads
Reactions
When tert-butyl chloride is dissolved in water, it undergoes a hydrolysis to tert-butyl alcohol. When dissolved in alcohols, the corresponding t-butyl ethers are produced.
Uses
tert-Butyl chloride is used to prepare the antioxidant tert-butylphenol and the fragrance neohexyl chloride.[1]
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads




