Top Qs
Timeline
Chat
Perspective
Thymolphthalexone
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
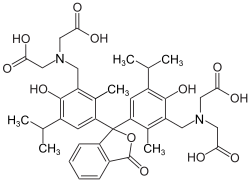
Thymolphthalexone is a chemical compound from the group of iminodiacetic acid derivatives of thymolphthalein.[3] Its chemical formula is C38H44N2O12.
This is a metallochromic indicator widely used in complexometric titrations, particularly for the determination of transition metals. The compound features a thymolphthalein-derived core linked to aminopolycarboxylic acid functional groups. This hybrid architecture grants the compound the ability to preferentially bind specific metal ions through coordinated interactions.
Remove ads
Synthesis
Thymolphthalexone can be obtained by Mannich condensation of formaldehyde and iminodiacetic acid with thymolphthalein.[4]
Physical properties
Thymolphthalexone forms a white crystalline powder soluble in water and organic solvents.[2]
Uses
Thymolphthalexone and its sodium salt are used as an indicator or photometric reagent for alkaline metal ions, such as those of calcium, strontium, barium, and others.[5][6]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads