Top Qs
Timeline
Chat
Perspective
Tin(IV) fluoride
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Tin(IV) fluoride is a chemical compound of tin and fluorine with the chemical formula SnF4. It is a white solid. As reflected by its melting point above 700 °C, the tetrafluoride differs significantly from the other tetrahalides of tin.[1]
Remove ads
Synthesis and reaction
SnF4 can be prepared by the reaction of tin(IV) chloride with anhydrous hydrogen fluoride:[1]
- SnCl4 + 4HF → SnF4 + 4HCl
When treated with alkali metal fluorides (e.g. KF), tin(IV) fluoride forms hexafluorostannates:
- SnF4 + 2 KF → K2SnF6
In K2SnF6, tin adopts an octahedral geometry.
Otherwise, SnF4 behaves as a Lewis acid forming a variety of adducts with the formula L2·SnF4 and L·SnF4.[2]
Structure
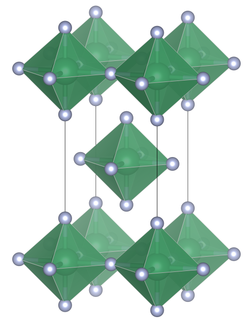
Unlike the heavier tin tetrahalides, which contain tetrahedrally coordinated tin, tin(IV) fluoride contains octahedrally coordinated tin. The octahedra share four corners. There are two terminal, unshared, fluorine atoms trans to one another.[3] The melting point of SnF4 is much higher (700 °C) than the other tin(IV) halides: (SnCl4, −33.3 °C; SnBr4, 31 °C; SnI4, 144 °C).[1] The structure can also be contrasted with the tetrafluorides of the lighter members of group 14, (CF4, SiF4 and GeF4), all of which in the solid state form molecular crystals.[2]
Remove ads
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads