Top Qs
Timeline
Chat
Perspective
VapBC
From Wikipedia, the free encyclopedia
Remove ads
VapBC (virulence associated proteins B and C) is the largest family of type II toxin-antitoxin system genetic loci in prokaryotes.[1] VapBC operons consist of two genes: VapC encodes a toxic PilT N-terminus (PIN) domain, and VapB encodes a matching antitoxin.[2] The toxins in this family are thought to perform RNA cleavage, which is inhibited by the co-expression of the antitoxin, in a manner analogous to a poison and antidote.
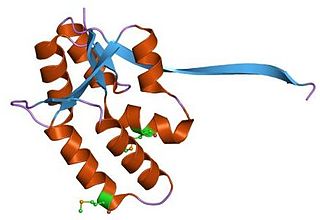
First discovered in 1992, vapBC loci are now thought make up around 37–42% of all type II toxin-antitoxin systems.[3][4]
Remove ads
Discovery
Following the discoveries of two other type II toxin-antitoxin systems,[5][6] the first vapBC system to be characterised was found in Salmonella dublin strain G19 in 1992.[7] It was characterised as a system for ensuring that all daughter cells contained a copy of the plasmid encoding the vapBC locus. The two components of this plasmidic system were originally named vagC and vagD (virulence-associated gene) for the toxin and antitoxin genes respectively. VagC was predicted to encode a 12kDa polypeptide, while vagD encoded a smaller 10kDa protein.[7] Their open reading frames were found to overlap by a single nucleotide; suggesting they were translated together, and at a constant molar ratio.[8]
Remove ads
Distribution
VapBC operons have been found in distantly related prokaryotes, including the pathogens Leptospira interrogans,[9] Mycobacterium tuberculosis[10] and Piscirickettsia salmonis.[11] The loci have been described as "surprisingly abundant, especially in Archaea"[12]—vapBC family members made up 37% of all TA families identified by one bioinformatics search[3] and 42% of those found by another.[4]
Bioinformatics searches have discovered vapBC homologues on both chromosomes and plasmids, and often in high copy number per cell. They are less common, however, in Bacillota and "Cyanobacteria".[3] Genomes with high numbers of vapBC loci include: M. tuberculosis with 45 predicted loci;[10] S.tokodaii with 25;[4] S.solfataricus with 23[4] and Sinorhizobium meliloti with 21.[10]
Remove ads
Function(s)

VapC toxins, specifically the PIN domains, act as ribonucleases in cleaving RNA molecules, thereby reducing the rate of translation.[10][14] In the bacteria Shigella flexneri and Salmonella enterica, VapC toxins have been shown to perform specific cleavage of a tRNA, but in other bacteria the RNA cleavage may be less specific.[15] The specificity of VapC-mediated RNase activity is thought to be influenced by both the primary sequence of the target and secondary structural motifs.[16]
VapC is strongly inhibited by direct protein interaction with VapB, its cognate antitoxin. The toxin-antitoxin complex is thought to autoregulate its own operon, repressing transcription of both components through a DNA-binding domain in VapB.[17]
In some organisms, vapBC loci have been assigned other potential functions. In the hyperthermophilic archaean Sulfolobus solfataricus, for example, a vapBC gene cassette is thought to regulate heat shock response.[2]
See also
References
Further reading
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads
