Top Qs
Timeline
Chat
Perspective
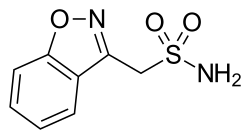
Zonisamide
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Zonisamide, sold under the brand name Zonegran among others, is a medication used to treat the symptoms of epilepsy and Parkinson's disease.[6][7] Chemically it is a sulfonamide. It serves as an anticonvulsant used primarily as an adjunctive therapy in adults with Parkinson's disease, partial-onset seizures; infantile spasm, mixed seizure types of Lennox–Gastaut syndrome, myoclonic and generalized tonic clonic seizure.[8] Despite this it is also sometimes used as a monotherapy for partial-onset seizures.[7][9]
In 2020, it was the 276th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[10][11]
Remove ads
Medical uses
Epilepsy
Zonisamide is approved in the United States,[2][12] and United Kingdom[13] for adjunctive treatment of partial seizures in adults and Japan for both adjunctive and monotherapy for partial seizures (simple, complex, secondarily generalized), generalized (tonic, tonic-clonic (grand mal), and atypical absence) and combined seizures.[14] In Australia it is marketed as both an adjunctive therapy and monotherapy for partial seizures only.[9]
Parkinson's disease
It has been approved for the treatment of the motor symptoms of Parkinson's disease (PD), as an adjunct to levodopa, in a few countries such as Japan.[6][7] In Japan, zonisamide has been used as an adjunct to levodopa treatment since 2009.[15] In addition, there is clinical evidence that zonisamide in combination with levodopa control of motor symptoms of PD but evidence for the treatment of the non motor symptoms of PD lacking.[16][17]
Remove ads
Adverse effects
Summarize
Perspective
Adverse effects by incidence:[5][18][19]
Very common (>10% incidence) adverse effects include:
- Anorexia
- Somnolence
- Dizziness
- Agitation
- Irritability
- Confusional state
- Depression
- Diplopia
- Memory impairment
- Decreased bicarbonate
Common (1–10% incidence) adverse effects include:
- Ecchymosis
- Hypersensitivity
- Affect lability
- Anxiety
- Insomnia
- Psychotic disorder
- Bradyphrenia
- Disturbance in attention
- Nystagmus
- Paraesthesia
- Speech disorder
- Tremor
- Abdominal pain
- Constipation
- Diarrhoea
- Dyspepsia
- Nausea
- Rash
- Pruritus
- Alopecia
- Nephrolithiasis
- Fatigue
- Influenza-like illness
- Pyrexia
- Oedema peripheral
- Weight loss
Incidence unknown
- Reproductive toxic effects[20]
Interactions
Zonisamide and other carbonic anhydrase inhibitors such as topiramate, furosemide, and hydrochlorothiazide have been known to interfere with amobarbital, which has led to inadequate anesthetization during the Wada test.[21] Zonisamide may also interact with other carbonic anhydrase inhibitors to increase the potential for metabolic acidosis.[5]
Additionally, the metabolism of zonisamide is inhibited by ketoconazole, ciclosporin, miconazole, fluconazole and carbamazepine (in descending order of inhibition) due to their effects on the CYP3A4 enzyme.[22]
Zonisamide is not known to inhibit cytochrome P450 enzymes when present at therapeutic concentrations.[23]
Remove ads
Mechanism of action
Zonisamide is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The precise mechanism by which zonisamide exerts its antiseizure effect is unknown, although it is believed that the drug blocks sodium and T-type calcium channels, which leads to the suppression of neuronal hypersynchronization (that is, seizure-form activity).[9] It is also known to be a weak carbonic anhydrase inhibitor (similarly to the anticonvulsant topiramate). It is also known to modulate GABAergic and glutamatergic neurotransmission.[9][24][25][26][27]
Pharmacokinetics
Absorption
Variable, yet relatively rapid rate of absorption with a time to peak concentration of 2.8–3.9 hours. Bioavailability is close to 100% and food has no effect on the bioavailability of zonisamide but may affect the rate of absorption.[28][23]
Metabolism
Zonisamide is metabolized mostly by the CYP3A4 isoenzyme, but also CYP3A7 and CYP3A5,[29] to 2-(sulphamoylacetyl)-phenol via reductive cleavage of the 1,2-benzisoxazole ring.[30]
Remove ads
History
Zonisamide was discovered by Uno and colleagues in 1972[31] and launched by Dainippon Sumitomo Pharma (formerly Dainippon Pharmaceutical) in 1989 as Excegran in Japan.[32] It was marketed by Élan in the United States starting in 2000 as Zonegran, before Élan transferred their interests in zonisamide to Eisai Co., Ltd. in 2004.[33] Eisai also markets Zonegran in Asia (China, Taiwan, and fourteen others)[34] and Europe (starting in Germany and the United Kingdom).[35]
Remove ads
Research
Tardive dyskinesia
In an open-label trial zonisamide attenuated the symptoms of tardive dyskinesia.[36]
Obesity
It has also been studied for obesity[37] with significant positive effects on body weight loss and there are three ongoing clinical trials for this indication.[38][39][40] It was to be sold, when combined with bupropion, under the brand name Empatic, until its development was discontinued.[41]
Migraine
Zonisamide has been studied for and used as a migraine preventative medication, when topiramate is either ineffective or cannot be continued due to side effects.[7]
Bipolar depression
It has also been used off-label by psychiatrists as a mood stabilizer to treat bipolar depression.[42][43]
Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads