Top Qs
Timeline
Chat
Perspective
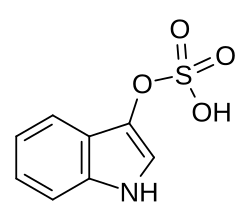
Indoxyl sulfate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Indoxyl sulfate, also known as 3-indoxylsulfate and 3-indoxylsulfuric acid, is a metabolite of dietary L-tryptophan that acts as a cardiotoxin and uremic toxin.[1][2][3] High concentrations of indoxyl sulfate in blood plasma are known to be associated with the development and progression of chronic kidney disease and vascular disease in humans.[1][2][3] As a uremic toxin, it stimulates glomerular sclerosis and renal interstitial fibrosis.[1][2]
Remove ads
Biosynthesis
Summarize
Perspective
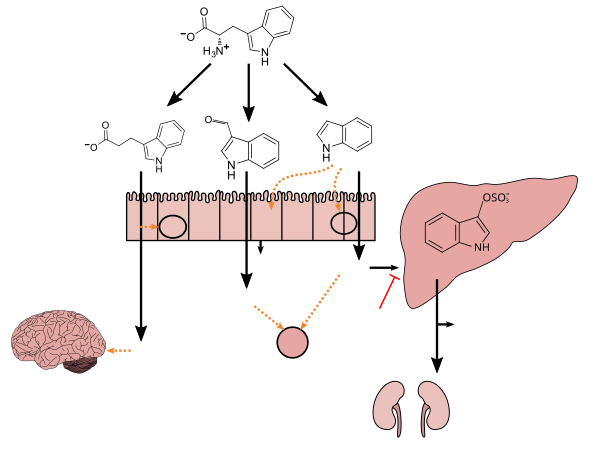
Indoxyl sulfate is a metabolite of dietary L-tryptophan that is synthesized through the following metabolic pathway:[3][4][5]
Indole is produced from L-tryptophan in the human intestine via tryptophanase-expressing gastrointestinal bacteria.[3] Indoxyl is produced from indole via enzyme-mediated hydroxylation in the liver;[3][4] in vitro experiments with rat and human liver microsomes suggest that the CYP450 enzyme CYP2E1 hydroxylates indole into indoxyl.[4] Subsequently, indoxyl is converted into indoxyl sulfate by sulfotransferase enzymes in the liver;[4][5] based upon in vitro experiments with recombinant human sulfotransferases, SULT1A1 appears to be the primary sulfotransferase enzyme involved in the conversion of indoxyl into indoxyl sulfate.[5]
Tryptophan metabolism by human gut microbiota ()
|
Remove ads
Clinical significance
Occasionally in urinary tract infections, bacteria produce indoxyl phosphatase which splits indoxyl sulfate forming indigo and indirubin creating dramatic purple urine.[9] Indoxyl sulfate is also a product of indole metabolism, which is produced from tryptophan by intestinal flora, such as Escherichia coli.[10]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads