Top Qs
Timeline
Chat
Perspective
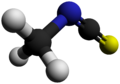
Methyl isothiocyanate
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Methyl isothiocyanate is the organosulfur compound with the formula CH3−N=C=S. This low melting colorless solid is a powerful lachrymator. As a precursor to a variety of valuable bioactive compounds, it is the most important organic isothiocyanate in industry.[1]
Remove ads
Synthesis
It is prepared industrially by two routes. Annual production in 1993 was estimated to be 4,000 tonnes.[citation needed] The main method involves the thermal rearrangement of methyl thiocyanate:[1]
- CH3−S−C≡N → CH3−N=C=S
It is also prepared via with the reaction of methylamine with carbon disulfide followed by oxidation of the resulting dithiocarbamate with hydrogen peroxide. A related method is useful to prepare this compound in the laboratory.[2]
MITC forms naturally upon the enzymatic degradation of glucocapparin, a glucoside found in capers.
Remove ads
Reactions
A characteristic reaction is with amines to give methyl thioureas:
- CH3NCS + R2NH → R2NC(S)NHCH3
Other nucleophiles add similarly.
Applications
Solutions of MITC are used in agriculture as soil fumigants, mainly for protection against fungi and nematodes.[3]
MITC is a building block for the synthesis of 1,3,4-thiadiazoles, which are heterocyclic compounds used as herbicides. Commercial products include "Spike", "Ustilan," and "Erbotan."
Well known pharmaceuticals prepared using MITC include Zantac and Tagamet. Suritozole is a third example.
MITC is used in the Etasuline patent (Ex2[4]), although the compound is question (Ex6) is with EITC.
Safety
MITC is a dangerous lachrymator as well as being poisonous.
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads