Platinum(IV) bromide is the inorganic compound with the formula PtBr4. It is a brown solid. It is a little-used compound mainly of interest for academic research.[2] It is a component of a reagent used in qualitative inorganic analysis.[3]
Quick facts Names, Identifiers ...
Platinum(IV) bromide
 |
| Names |
| IUPAC name
Platinum(IV) bromide |
| Other names
Platinic bromide |
| Identifiers |
|
|
|
|
| ChemSpider |
|
| ECHA InfoCard |
100.066.481 |
| EC Number |
|
|
|
|
|
InChI=1S/4BrH.Pt/h4*1H;/q;;;;+4/p-4 Key: SNPHNDVOPWUNON-UHFFFAOYSA-J
|
|
| Properties |
|
PtBr4 |
| Molar mass |
514.694 g/mol |
| Appearance |
brownish-black crystals |
| Melting point |
decomposes at 180°C |
|
0.41 g/100mL @ 20°C |
| Solubility |
slightly soluble in ethanol, diethyl ether[1] |
| Hazards |
| GHS labelling: |
|
 |
|
Danger |
|
H314 |
|
P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 |
| NFPA 704 (fire diamond) |
|
| Flash point |
non-flammable |
| Safety data sheet (SDS) |
|
| Related compounds |
|
Platinum(IV) fluoride
Platinum(IV) chloride
Platinum(IV) iodide |
|
Nickel(II) bromide
Palladium(II) bromide |
Related compounds |
Platinum(II) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Close
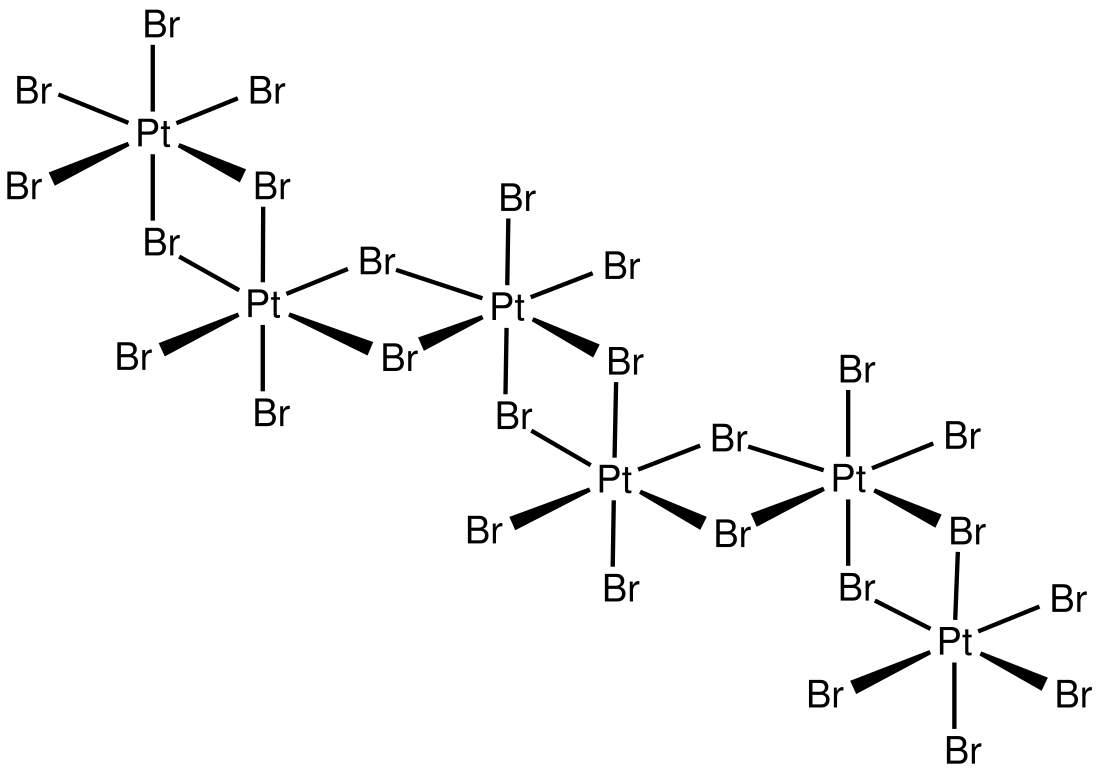
In terms of structure, the compound is an inorganic polymer consisting of interconnected PtBr6 octahedra.