Top Qs
Timeline
Chat
Perspective
Substituted cathinone
Class of chemical compounds From Wikipedia, the free encyclopedia
Remove ads
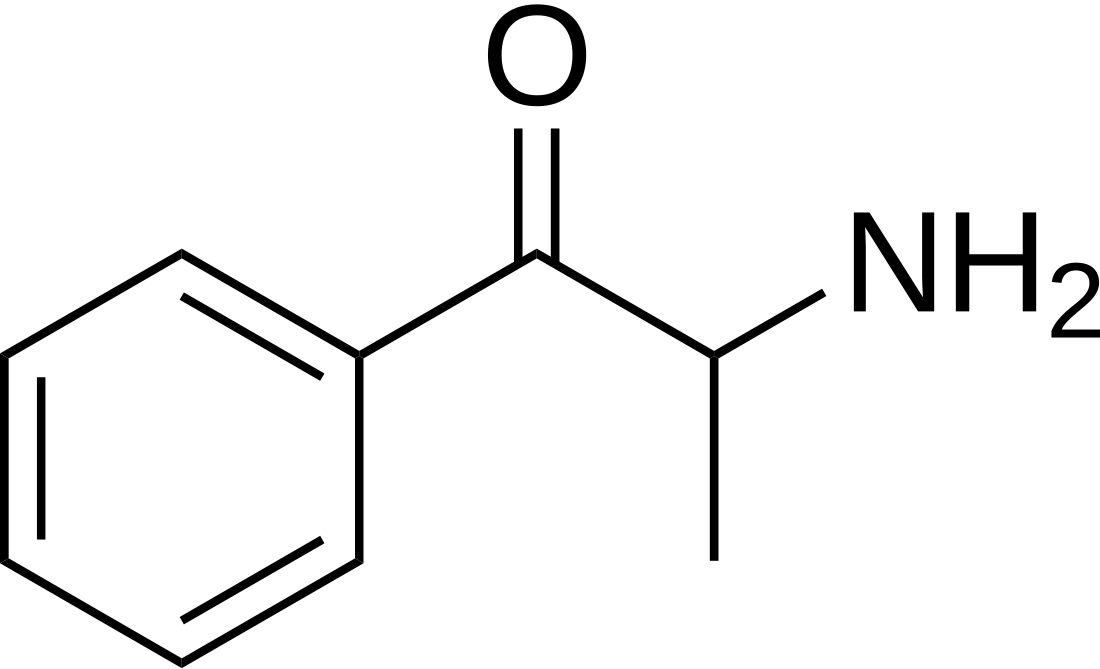
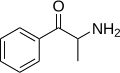
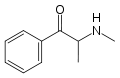
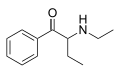
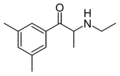
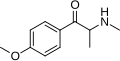
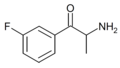
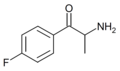
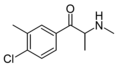
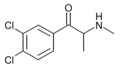
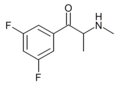
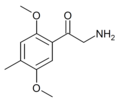
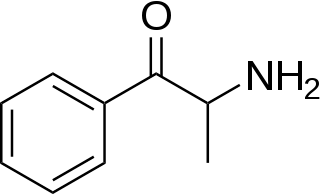
Substituted cathinones, or simply cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions.[1][2][3][4][5] Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.[6]
Substituted cathinones act as monoamine releasing agents and/or monoamine reuptake inhibitors, including of norepinephrine, dopamine, and/or serotonin.[7][8][9][10][11][12] In contrast to substituted amphetamines, most substituted cathinones do not act as agonists of the human trace amine-associated receptor 1 (TAAR1).[13][14][15] This may potentiate their stimulating and addictive effects.[13][14] In addition, β-keto-substituted phenethylamines, such as βk-2C-B, appear to show dramatically reduced potency and efficacy as serotonin 5-HT2A receptor agonists compared to their non-β-keto-substituted counterparts.[16]
Remove ads
Monoamine release profiles
Summarize
Perspective
The following is a list of serotonin, dopamine, and norepinephrine releasing profiles for various cathinones, measured in rat brain synaptosomes.[7][8][17]
Remove ads
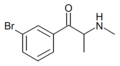
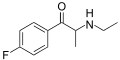
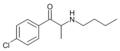
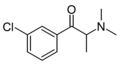
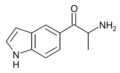
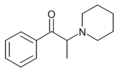
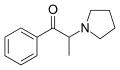
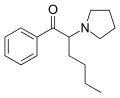
List of substituted cathinones
Summarize
Perspective
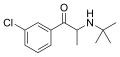
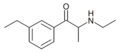
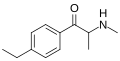
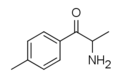
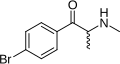
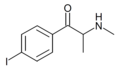
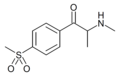
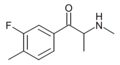
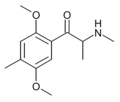
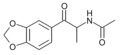
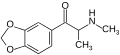
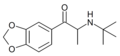
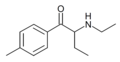
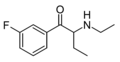
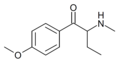
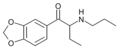
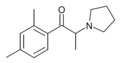
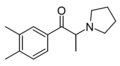
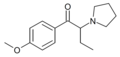
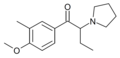
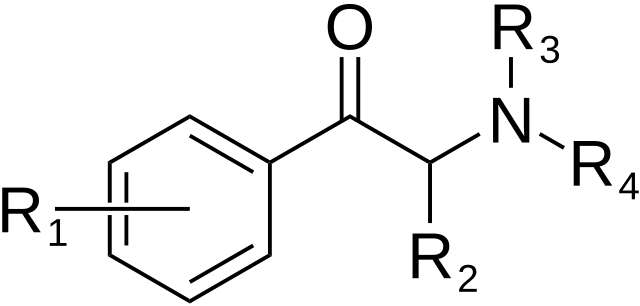
The derivatives may be produced by substitutions at four locations of the cathinone molecule:
- R1 = hydrogen, or any combination of one or more alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents
- R2 = hydrogen or any alkyl group
- R3 = hydrogen, any alkyl group, or incorporation in a cyclic structure
- R4 = hydrogen, any alkyl group, or incorporation in a cyclic structure
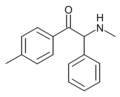
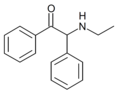
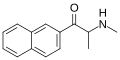
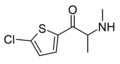
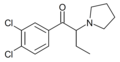
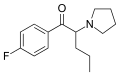
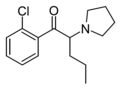
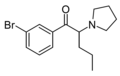
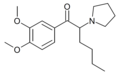
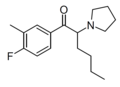
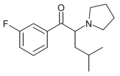
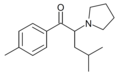
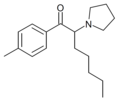
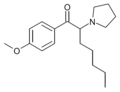
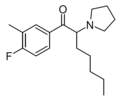
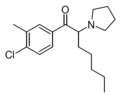
The following table displays notable derivatives that have been reported:[55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76]
Remove ads
Legality
Summarize
Perspective
On 2 April 2010, the Advisory Council on the Misuse of Drugs in the UK announced that a broad structure-based ban of this entire class of compounds would be instituted, following extensive publicity around grey-market sales and recreational use of mephedrone, a common member of the family. This ban covers compounds with the aforementioned general structure, with 28 compounds specifically named.[77]
"Any compound (not being bupropion or a substance for the time being specified in paragraph 2.2) structurally derived from 2-amino-1-phenyl-1-propanone by modification in any of the following ways, that is to say,
(i) by substitution in the phenyl ring to any extent with alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents, whether or not further substituted in the phenyl ring by one or more other univalent substituents;
(ii) by substitution at the 3-position with an alkyl substituent;
(iii) by substitution at the nitrogen atom with alkyl or dialkyl groups, or by inclusion of the nitrogen atom in a cyclic structure."
— ACMD, 2 April 2010
This text was added as an amendment to the Misuse of Drugs Act 1971, to come into force on 16 April 2010.[78] Note that four of the above compounds (cathinone, methcathinone, diethylpropion and pyrovalerone) were already illegal in the UK at the time the ACMD report was issued. Two compounds were specifically excluded from the ban, these being bupropion because of its common use in medicine and relative lack of abuse potential, and naphyrone because its structure falls outside the generic definition and not enough evidence was yet available to justify a ban.
Naphyrone analogues were subsequently banned in July 2010 following a further review by the ACMD,[79][80] along with a further broad based structure ban even more expansive than the last.[81][82]
"Any compound structurally derived from 2–aminopropan–1–one by substitution at the 1-position with any monocyclic, or fused-polycyclic ring system (not being a phenyl ring or alkylenedioxyphenyl ring system), whether or not the compound is
further modified in any of the following ways, that is to say—
(i) by substitution in the ring system to any extent with alkyl, alkoxy, haloalkyl or halide substituents, whether or not further substituted in the ring system by one or more other univalent substituents;
(ii) by substitution at the 3–position with an alkyl substituent;
(iii) by substitution at the 2-amino nitrogen atom with alkyl or dialkyl groups, or
by inclusion of the 2-amino nitrogen atom in a cyclic structure".
— Home Office, 13 July 2010.

The substitutions in the general structure for naphyrone analogues subject to the ban may be described as follows:
- Cyc = any monocyclic, or fused-polycyclic ring system (not being a phenyl ring or alkylenedioxyphenyl ring system), including analogues where the ring system is substituted to any extent with alkyl, alkoxy, haloalkyl or halide substituents, whether or not further substituted in the ring system by one or more other univalent substituents
- R1 = hydrogen or any alkyl group
- R2 = hydrogen, any alkyl group, or incorporation in a cyclic structure
- R3 = hydrogen, any alkyl group, or incorporation in a cyclic structure
More new derivatives have however continued to appear, with the UK reporting more novel cathinone derivatives detected in 2010 than any other country in Europe, with most of them first identified after the generic ban had gone into effect and thus already being illegal despite never having been previously reported.[83]
In the United States, substituted cathinones are the psychoactive ingredients in "bath salts" which as of July 2011 were banned by at least 28 states, but not by the federal government.[84]
Remove ads
See also
- Markush structure
- Substituted amphetamines
- Substituted β-hydroxyamphetamines
- Substituted methylenedioxyphenethylamines
- Substituted phenethylamines
- Substituted phenylmorpholines
- Substituted β-ketotryptamines
- Structural scheduling of synthetic cannabinoids
- Arylcyclohexylamines
- List of aminorex analogues
- List of fentanyl analogues
- List of methylphenidate analogues
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads