Top Qs
Timeline
Chat
Perspective
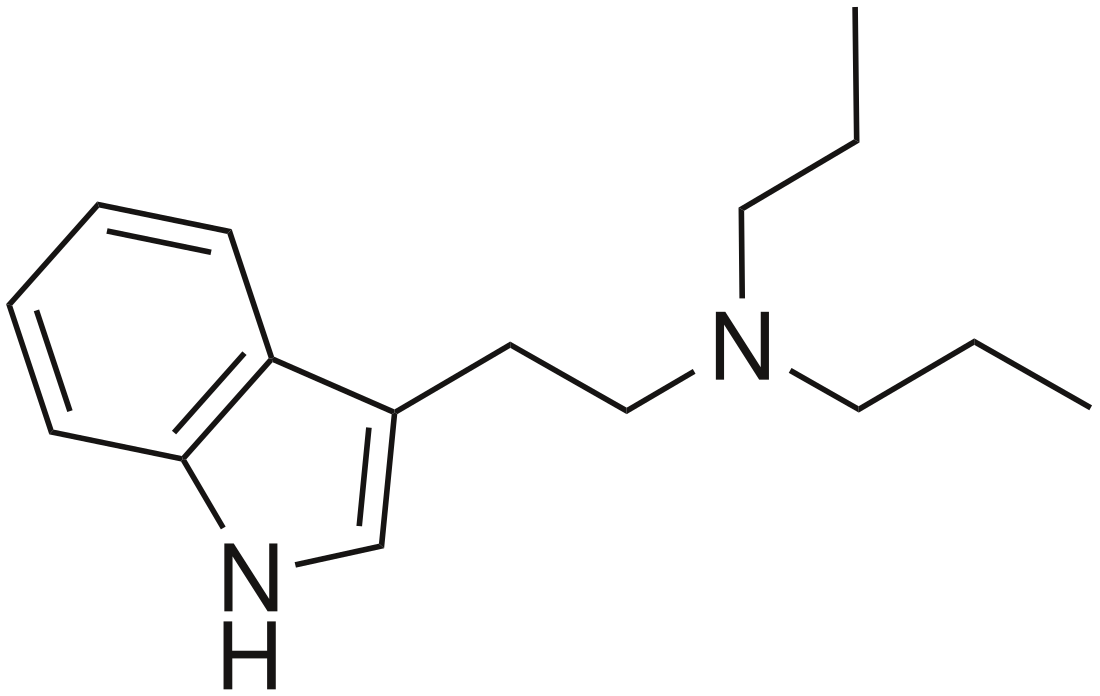
Dipropyltryptamine
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Dipropyltryptamine (DPT), also known as N,N-dipropyltryptamine or as "The Light", is a psychedelic drug of the tryptamine family related to dimethyltryptamine (DMT).[1][3] It is taken orally or by other routes.[1]
The drug acts as a serotonin receptor modulator, including as a serotonin 5-HT2A receptor agonist.[4][5][6][7][8] It is a close structural homologue of DMT and diethyltryptamine (DET).[1] Derivatives of DPT include 4-HO-DPT and 5-MeO-DPT, among others.[1]
DPT was first described in the literature by 1959.[9][10][11] It was encountered as a novel designer drug by 1968[12] and was reported as a possible treatment for alcoholism in 1973.[2][3][13] The drug is the sacrament of the Temple of the True Inner Light, a New York City-based religious group.[1][14][15]
Remove ads
Use and effects
Summarize
Perspective
In his book TiHKAL (Tryptamines I Have Known and Loved), Alexander Shulgin lists DPT's dose range as 100 to 250 mg orally and its duration as 2 to 4 hours.[1][2] A 500 mg dose was also reported, which was described as "exhausting" and as lasting 12 hours.[1] The onset and time to peak effects were not given, but it was implied to have a fast onset.[1] In addition to oral administration, DPT has been assessed by smoking at a dose of 100 mg, by intramuscular injection at low doses of 15 to 30 mg, moderate doses of 30 to 70 mg, and "peak experience" doses of 75 to 125 mg, and by intravenous injection at 12 to 36 mg.[1][16]
The effects of DPT have been reported to include visuals, being intensely visual at high doses, changes in time perception, feeling like one is in a different place like on a mountain in clouds or in a big castle, enhanced recall of memories and experiences, enhanced emotional expressiveness and self-exploration, entity encounters, and religious feelings.[1][17][13] Other effects included trouble talking, feeling uncomfortable, nervousness, feeling light, and body rush.[1] Given at a high dose intravenously, it was described as every bit as powerful as a psychedelic as DMT.[1] According to one account however, DPT and DMT, despite their chemical similarity, "reveal completely different worlds".[17]
Other reports have stated effects of DPT including visual and auditory hallucinations, increased color intensity, flashes of light and sparkles, apparitions of faces, increased music appreciation, ego dissolution, stimulation, euphoria, relaxation, paranoia, psychosis, anxiety, nausea, dizziness, muscle tremors, and increased heart rate, among others.[2] Its duration is described as much shorter than those of certain other psychedelics like LSD, which can be advantageous in a clinical setting.[18][19][20] However, it is also said to have a rapid onset that can be psychologically overwhelming.[18][20]
Remove ads
Side effects
Although tryptamines such as psilocybin and dimethyltryptamine (DMT) have relatively well‑characterized safety, synthetic analogues like DPT lack thorough toxicological evaluation and are mainly associated with anecdotal reports of intoxication and a few cases of fatal outcomes when used recreationally.[21] The pharmacological similarity of DPT to DMT suggests a generally low intrinsic toxicity at controlled doses but a pronounced risk of acute adverse reactions, including agitation, tachycardia, hyperthermia, and serotonergic crisis, particularly in combination with monoamine oxidase inhibitors or other serotonergic substances.[21]
A meta-analysis of tryptamine psychedelics have further demonstrated cognitive effects through serotonin 5-HT2A receptor modulation but have not identified persistent neurotoxicity.[22] The main safety concerns are acute psychophysiological and behavioral disturbances rather than long‑term organ toxicity. Overall, DPT is a potent, short‑acting serotonergic hallucinogen with limited safety data and a toxicity profile comparable to related tryptamines such as DMT and 5-MeO-DMT.[21][22]
Remove ads
Interactions
Pharmacology
Summarize
Perspective
Pharmacodynamics
DPT produces the head-twitch response, a behavioral proxy of psychedelic-like effects, in rodents.[16][25] Studies on rodents have found that the effectiveness with which a selective 5-HT2A receptor antagonist blocks the behavioral actions of DPT strongly suggests that the 5-HT2A receptor is an important site of action for the drug, but the modulatory actions of a serotonin 5-HT1A receptor antagonist also imply a serotonin 5-HT1A receptor-mediated component to the actions of DPT.[25] Unusually among most psychedelics, DiPT did not show evidence of behavioral tolerance in rodents.[26]
Remove ads
Chemistry

DPT, also known as N,N-dipropyltryptamine, is a substituted tryptamine related to dimethyltryptamine (DMT).[1] It is found either as a crystalline hydrochloride salt or as an oily or crystalline base. The drug is synthetic and has not been found to occur endogenously.[17]
Detection
DPT changes Ehrlich's reagent violet and causes the marquis reagent to turn yellow.[27]
Synthesis
The chemical synthesis of DPT has been described.[1]
Analogues
Analogues of DPT include dimethyltryptamine (DMT), diethyltryptamine (DET), diisopropyltryptamine (DiPT), diallyltryptamine (DALT), methylethyltryptamine (MET), methylpropyltryptamine (MPT), ethylpropyltryptamine (EPT), propylisopropyltryptamine (PiPT), propylallyltryptamine (PALT), 4-HO-DPT, 5-HO-DPT, and 5-MeO-DPT, among others.[1]
Remove ads
History
DPT was first described in the scientific literature by 1959.[9][10][11] Use of DPT as a designer drug has been documented by law enforcement officials since as early as 1968.[12] It was described as a treatment for alcoholism by Stanislav Grof and colleagues in 1973.[3][2][13][28] It was also studied for treatment of anxiety associated with terminal cancer in the late 1970s.[28] However, it was not further studied for such purposes after 1980.[29]
Remove ads
Society and culture
Religious use
DPT is used as a religious sacrament by the Temple of the True Inner Light, a New York City offshoot of the Native American Church.[1] The Temple believes DPT and other entheogens are physical manifestations of God.[1][30]
Legal status
Sweden
DPT is illegal in Sweden as of 26 January 2016.[31]
United Kingdom
DPT is a Class A drug in the United Kingdom, making it illegal to possess or distribute.
United States
DPT is not scheduled at the federal level in the United States,[32] but it could be considered an analog of 5-MeO-DiPT, DMT, or DET, in which case purchase, sale, or possession could be prosecuted under the Federal Analogue Act.
Florida
"DPT (N,N-Dipropyltryptamine)" is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.[33]
Maine
DPT is a Schedule I controlled substance in the state of Maine making it illegal to buy, sell, or possess in Maine.
Remove ads
Research
Fragile X syndrome
DPT has been found to completely prevent audiogenic seizures in mouse models of fragile X syndrome (FXS) at a 10 mg/kg dose, with its mechanism of action appearing to be independent of serotonin and sigma σ1 receptor activation.[34] While DPT is an agonist at several serotonin receptors in vitro, its anticonvulsant effects were not blocked by selective serotonin 5-HT2A, 5-HT1A, or 5-HT1B receptor antagonists nor by a selective sigma σ1 receptor antagonist in vivo.[34] The drug's beneficial effects may be mediated by non-serotonergic pathways, possibly involving direct auditory processing modulation.[34] At higher doses, DPT switched from anticonvulsant to proconvulsant action, indicating complex interactions.[34]
Remove ads
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads