Top Qs
Timeline
Chat
Perspective
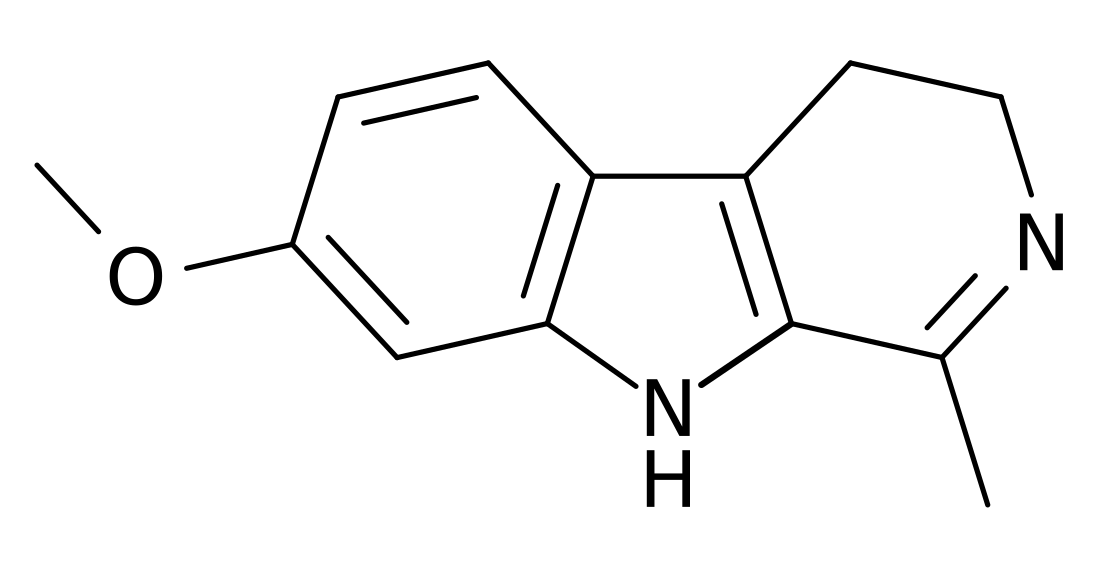
Harmaline
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Harmaline, also known as 7-methoxyharmalan or as 3,4-dihydro-7-methoxy-1-methyl-β-carboline, is a harmala alkaloid and β-carboline which has hallucinogenic effects and monoamine oxidase inhibitor (MAOI) activity.[4][2][1] It is the partly hydrogenated form of harmine.[4][1]
Plants containing harmaline are combined in ayahuasca to inhibit monoamine oxidase, allowing orally consumed dimethyltryptamine (DMT) to become orally active and produce psychoactive effects. Harmala alkaloids, including harmaline, are psychoactive on their own in humans, with harmaline being particularly hallucinogenic, although other compounds such as harmine and tetrahydroharmine have also been reported to produce hallucinogenic effects as well.
Harmaline is present in Peganum harmala (Syrian rue). Syrian rue seeds contain about 3% harmala alkaloids by dry weight. Harmaline was first isolated from plants in 1841, its chemical structure identified in 1919, and it was first synthesized in 1927.
Remove ads
Use and effects
Summarize
Perspective
As a hallucinogen
The harmala alkaloids are psychoactive in humans.[5] According to Alexander Shulgin, harmaline is the only harmala alkaloid that has a reputation of being hallucinogenic.[2][6] However, other harmala alkaloids and β-carbolines, like harmine, tetrahydroharmine (THH), 6-methoxyharmalan, and 6-methoxytetrahydroharman, have also been reported to be hallucinogenic.[7] Harmaline produces vivid dream-like visual effects and physical discomfort at doses of 150 to 400 mg orally or 70 to 100 mg intravenously, often leading users to seek solitude in a quiet, dark environment.[2][1] The hallucinogenic effects of harmaline and other β-carbolines are said to be qualitatively distinct from and unlike those of serotonergic psychedelics like LSD but similar to those of ibogaine.[8][9][10][11] Taken orally, the onset of harmaline is 1 to 2 hours, peak effects occur after around 2 hours, and its duration is 5 to 8 hours.[1][2] Conversely, its onset by intravenous injection is within seconds and its duration is much shorter by this route than with oral administration.[2]
As an MAOI
Harmaline is a monoamine oxidase inhibitor (MAOI), or more specifically a reversible inhibitor of monoamine oxidase A (RIMA).[12][1] The effective doses for this activity are 70 to 150 mg orally.[1]
Harmaline-containing plants and tryptamine-containing plants are used in ayahuasca brews. The inhibitory effects on monoamine oxidase A (MAO-A) allows dimethyltryptamine (DMT), the psychoactively prominent chemical in the mixture, to bypass the extensive first-pass metabolism it undergoes upon ingestion, allowing a psychologically active quantity of the chemical to exist in the brain for a perceivable period of time.[13]
Besides DMT, harmaline has also been used to inhibit the metabolism of and thereby potentiate 5-MeO-DMT, which like DMT is otherwise orally inactive and has a very short duration.[1]
Remove ads
Interactions
Harmaline is a reversible inhibitor of MAO-A (RIMA)".[14] This means that the risk of a hypertensive crisis, a dangerous high blood pressure crisis from eating tyramine-rich foods such as cheese, is likely lower with harmaline than with irreversible MAOIs such as phenelzine. Since harmaline is a RIMA, it could, in theory, induce both serotonin syndrome and hypertensive crises in combination with tyramine, serotonergics, catecholaminergics drugs or prodrugs.
Remove ads
Pharmacology
Summarize
Perspective
Pharmacodynamics
Harmaline shows weak but significant affinity for the serotonin 5-HT2A and 5-HT2C receptors in the low micromolar range.[7][18] However, harmaline and other β-carbolines do not activate the serotonin 5-HT2A receptor even at very high concentrations in vitro.[16] Harmaline shows high affinity for the imidazoline I2 receptor (Ki = 22 nM).[17] Unlike ibogaine and noribogaine, harmaline does not bind to the κ-opioid receptor or other opioid receptors.[21]
Harmaline and the psychedelic DOM partially substitute for each other in rodent drug discrimination tests.[22][7] Harmaline was much more effective in substituting for DOM than harman and harmine, which did not achieve significant generalization and produced behavioral disruption at higher doses.[22] On the other hand, harmaline and 6-methoxyharman were comparable in terms of DOM substitution.[22] Unlike serotonergic psychedelics, ibogaine and harmala alkaloids like harmaline do not cause pupil dilation or increase blood pressure in humans.[2][8]
Harmaline and ibogaine have both been found to produce neurotoxicity against Purkinje cells in the cerebellum in rats that is mediated by upstream olivocerebellar pathway activation.[23][24][25][26] This may explain long-lasting motor deficits induced by ibogaine in these rats.[27] However, this phenomenon involves high doses of ibogaine and has not been observed with ibogaine in primates or humans.[27][28][29] In any case, the rodent findings are notable in that they further suggest that harmaline and ibogaine share a common mechanism of action.[23]
Pharmacokinetics
The elimination half-life of harmaline has been reported to be about 2 hours.[3]
Remove ads
Chemistry

Harmaline, also known as 7-methoxyharmalan or 3,4-dihydro-7-methoxy-1-methyl-β-carboline, is a β-carboline and a cyclized tryptamine analogue of 6-methoxy-DMT.
Properties
It is fluorescent under ultraviolet light.
Synthesis
The chemical synthesis of harmaline has been described.[1]
Analogues
Analogues of harmaline include harmine, tetrahydroharmine, harmalol, 5-methoxyharmalan, 6-methoxyharmalan, and ibogamine, among others.[1]
Remove ads
Natural occurrence
Various plants contain harmaline including Peganum harmala (Syrian rue) as well as the hallucinogenic beverage ayahuasca, which is traditionally brewed using Banisteriopsis caapi. Present at 3% by dry weight, the harmala alkaloids may be extracted from the Syrian rue seeds.[5]
History
Harmaline was first isolated from plants in 1841.[2] The chemical structure of harmaline was not correctly identified until 1919.[2] Harmaline was first synthesized in 1927.[2]
Society and culture
Summarize
Perspective
Legal status
The examples and perspective in this article may not represent a worldwide view of the subject. (January 2016) |
Australia
Harmala alkaloids are considered Schedule 9 prohibited substances under the Poisons Standard (October 2015).[30] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[30]
Canada
Harmaline and Harmalol are considered Schedule III controlled substances by the Controlled Drugs and Substances Act. Every person found to be in possession of a Schedule III drug is guilty of an indictable offence and liable to imprisonment for a term not exceeding three years; or for a first offence, guilty on summary conviction, to a fine not exceeding one thousand dollars or to imprisonment for a term not exceeding six months, or to both. Every person found to be trafficking a Schedule III drug is guilty of an indictable offence and liable to imprisonment for a term not exceeding ten years, or is guilty on summary conviction (first-time offenders) and liable to imprisonment for a term not exceeding eighteen months.[31]
Remove ads
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads