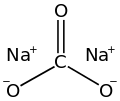
Skeletal formula of sodium carbonate Space-filling model of sodium carbonate
Names
IUPAC name
Sodium carbonate
Other names
Soda ash, Washing soda, Soda crystals
Identifiers
3D model (JSmol )
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.007.127
EC Number
E number E500(i) (acidity regulators, ...)
RTECS number
UNII
InChI
SMILES
Properties
Na2 CO3
Molar mass 105.9888 g/mol (anhydrous)
Appearance
White solid, hygroscopic
Odor Odorless
സാന്ദ്രത 2.54 g/cm3 (25 °C, anhydrous)3 (856 °C)3 (monohydrate)[ 1] 3 (heptahydrate)3 (decahydrate)[ 2]
ദ്രവണാങ്കം
Decahydrate: °C) °C) °C) °C) °C) °C) °C) °C)[ 3]
Solubility Soluble in aq. alkalis ,[ 3] glycerol alcohol CS2 , acetone , alkyl acetates , alcohol, benzonitrile , liquid ammonia [ 4]
Solubility in glycerine 98.3 g/100 g (15.5 °C)[ 4]
Solubility in ethanediol 3.46 g/100 g (20 °C)[ 5]
Solubility in dimethylformamide 0.5 g/kg[ 5]
Basicity (pK b )3.67
−4.1·10−5 cm3 /mol[ 2]
1.485 (anhydrous)[ 6]
വിസ്കോസിറ്റി 3.4 cP (887 °C)[ 5]
Structure
Monoclinic (γ-form, β-form, δ-form, anhydrous)[ 7] Orthorhombic (monohydrate, heptahydrate)[ 1] [ 8]
Space group
C2/m, No. 12 (γ-form, anhydrous, 170 K) K)1 /n, No. 14 (δ-form, anhydrous, 110 K)[ 7] 1 , No. 29 (monohydrate)[ 1] [ 8]
Point group
2/m (γ-form, β-form, δ-form, anhydrous)[ 7] [ 1] [ 8]
Lattice constant
a =
8.920(7)
Å,
b =
5.245(5)
Å,
c =
6.050(5)
Å (γ-form, anhydrous, 295
K)
[ 7] α = 90°, β = 101.35(8)°, γ = 90°
Octahedral (Na+ , anhydrous)
Thermochemistry
112.3 J/mol·K[ 2]
Std molar (S ⦵ 298 )
135 J/mol·K[ 2]
Std enthalpy of (Δf H ⦵ 298 )
−1130.7 kJ/mol[ 2] [ 5]
Gibbs free energy (Δ f G ⦵ )
−1044.4 kJ/mol[ 2]
Hazards
GHS labelling
Pictograms
[ 9]
Signal word
Warning
Hazard statements
H319 [ 9]
Precautionary statements
P305+P351+P338 [ 9]
NFPA 704 diamond)
Lethal dose or concentration (LD, LC):
LD50 (median dose )
4090 mg/kg (rat, oral) [ 11]
Safety data sheet (SDS)MSDS
Related compounds
Other anions
Sodium bicarbonate
Other cations
Lithium carbonate Potassium carbonate Rubidium carbonate Caesium carbonate
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| colspan=2 |
N verify (
what is :
Y /
N ?)