Top Qs
Timeline
Chat
Perspective
Lysergamides
Class of chemical compounds From Wikipedia, the free encyclopedia
Remove ads
Lysergamides, also known as ergoamides[1][2][3] or as lysergic acid amides, are amides of lysergic acid (LA). They are ergolines, with some lysergamides being found naturally in ergot as well as other fungi. Lysergamides are notable in containing embedded phenethylamine and tryptamine moieties within their ergoline ring system.[4]

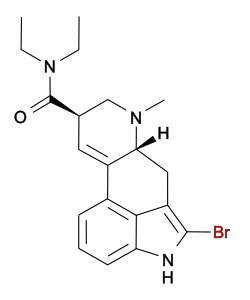
The simplest lysergamides are ergine (lysergic acid amide; LSA) and isoergine (iso-lysergic acid amide; iso-LSA). In terms of pharmacology, the lysergamides include numerous serotonin and dopamine receptor agonists, most notably the psychedelic drug lysergic acid diethylamide (LSD) but also a number of pharmaceutical drugs like ergometrine, methylergometrine, methysergide, and cabergoline.[5][6][7][8][9][10][11][12][13][14][15][16][17] Various analogues of LSD, such as the psychedelics ALD-52 (1A-LSD), ETH-LAD, LSZ, and 1P-LSD and the non-hallucinogenic 2-bromo-LSD (BOL-148), have also been developed. Ergopeptines like ergotamine, dihydroergotamine, and bromocriptine are also lysergamides, but with addition of a small peptide moiety at the amide. Close analogues of lysergamides that are not technically lysergamides themselves include lisuride, terguride, bromerguride, and JRT.
Lysergamides were first discovered and described in the 1930s.[18][19][20]
Simplified or partial ergolines and lysergamides, such as NDTDI (8,10-seco-LSD), DEMPDHPCA, and N-DEAOP-NMT, are also known.[21][22][23]
Remove ads
Use and effects
Summarize
Perspective
The doses, potencies, durations, and effects of lysergamides have been reviewed by Alexander Shulgin.[24][25][26][27][28] They have also been reviewed by Albert Hofmann,[29] David E. Nichols,[30] and other researchers.[31][32][33][34][35][36][37][38][39]
The properties of various additional lysergamides, for instance in terms of serotonin antagonism, have also been described.[50]
Remove ads
Interactions
Pharmacology
Pharmacodynamics
History
Summarize
Perspective

Lysergamides, such as ergine, isoergine, and ergometrine, were discovered by the early 1930s,[18][19][20] and LSD was discovered by 1938 and its hallucinogenic effects in 1943 by Albert Hofmann.[53][54] Many synthetic lysergamide analogues of LSD, modified at the amide and/or 1 or 2 positions, were first described by Hofmann and colleagues in the mid-to-late 1950s.[29][34][42][55] N(6)-Substituted lysergamides were first reported in 1970 and thereafter in the 1970s and 1980s by multiple groups, including Hofmann and colleagues, Yuji Nakahara and Tetsukichi Niwaguchi and colleagues, and David E. Nichols and colleagues.[56][57][58][6] The psychedelic effects of N(6)-substituted lysergamides were reported by Alexander Shulgin in 1986 and thereafter.[59][37][25][28] Additional novel lysergamides modified at the amide, like LA-3Cl-SB and LA-Aziridine, were described by Nichols and Robert Oberlender and colleagues in the late 1980s,[60][37][56] while LSZ (LA-Azetidine) was described by the same group in 2002.[9] Numerous 1-substituted LSD prodrugs such as 1P-LSD and 1V-LSD and other psychedelic lysergamides were developed by Lizard Labs in the 2010s and 2020s.[61][62][63]
Remove ads
List of lysergamides
Remove ads
Related compounds
Remove ads
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads