Ang arsenico (Iningles arsenic) mao ang elementong kimiko sa talaang peryodiko nga may simbolo nga As ug kaiphan nga atomik 33. Ang arsenico mao ang metalloid.
Quick facts Arsenic, Panagway ...
| Arsenic |
|---|
33As |
|
|
| Panagway |
metallic grey
 |
| Kinatibuk-ang mga kinaiya |
| Ngalan, simbolo, kaiphan |
arsenic, As, 33 |
| Paglitok |
[[Help:Pronunciation respelling key|Plantilya:Smallcaps all-sə-nik]],
also [[Help:Pronunciation respelling key|ar-Plantilya:Smallcaps all-ik]] when attributive |
| Kategoriyang elemento |
metalloid |
| Group, period, block |
15, 4, p |
| Gibug-aton sa atomo |
74.92160(2) |
| Kontorno sa elektron |
[Ar] 4s2 3d10 4p3
2, 8, 18, 5
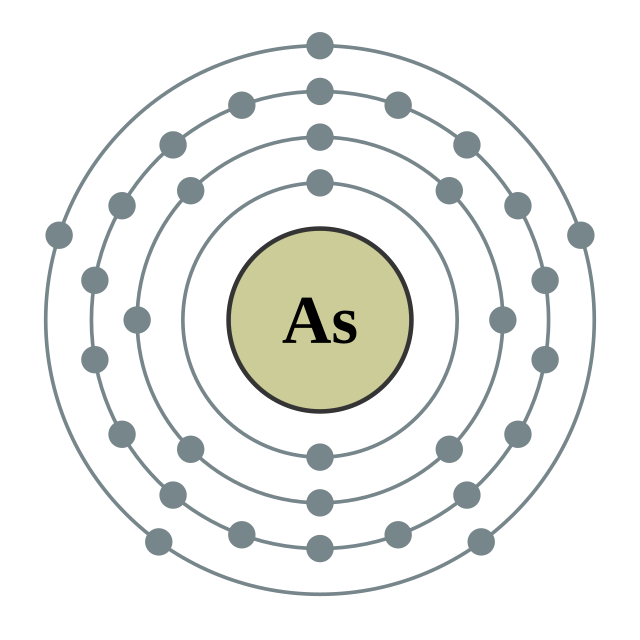 Electron shells of arsenic (2, 8, 18, 5) Electron shells of arsenic (2, 8, 18, 5) |
| History |
| Pagkadiskobre |
Early Bronze Age (2500 BC) |
| First isolation |
Albertus Magnus (1250) |
| Physical properties |
| Phase |
magahi |
| Density (near r.t.) |
5.727 g·cm−3 |
| Liquid density at m.p. |
5.22 g·cm−3 |
| Sublimation point |
887 K, 615 °C, 1137 °F |
| Triple point |
1090 K (817°C), 3628[1] kPa |
| Critical point |
1673 K, ? MPa |
| Heat of fusion |
(grey) 24.44 kJ·mol−1 |
| Heat of vaporization |
? 34.76 kJ·mol−1 |
| Molar heat capacity |
24.64 J·mol−1·K−1 |
| Vapor pressure |
| P (Pa) |
1 |
10 |
100 |
1 k |
10 k |
100 k |
| at T (K) |
553 |
596 |
646 |
706 |
781 |
874 |
|
| Atomic properties |
| Oxidation states |
5, 3, 2, 1,[2] -3
(mildly acidic oxide) |
| Electronegativity |
2.18 (Pauling scale) |
Ionization energies
(more) |
1st: 947.0 kJ·mol−1 |
| 2nd: 1798 kJ·mol−1 |
| 3rd: 2735 kJ·mol−1 |
| Atomic radius |
119 pm |
| Covalent radius |
119±4 pm |
| Van der Waals radius |
185 pm |
| Miscellanea |
| Crystal structure |
simple trigonal[3]
|
| Magnetic ordering |
diamagnetic[4] |
| Electrical resistivity |
(20 °C) 333 nΩ·m |
| Thermal conductivity |
50.2 W·m−1·K−1 |
| Young's modulus |
8 GPa |
| Bulk modulus |
22 GPa |
| Mohs hardness |
3.5 |
| Brinell hardness |
1440 MPa |
| CAS registry number |
7440-38-2 |
| Most stable isotopes |
| Main article: Isotopes of arsenic |
| iso |
NA |
half-life |
DM |
DE (MeV) |
DP
Plantilya:Infobox element/isotopes decay2 |
|---|
| 74As |
syn |
17.78 d |
ε |
74Ge |
| β+ |
74Ge |
| γ |
- |
| β− |
74Se
Plantilya:Infobox element/isotopes stable |
|
| · r |
Close



