Ang karbon o carbono (Iningles carbon) mao ang elementong kimiko sa talaang peryodiko nga may simbolo nga C ug kaiphan nga atomik 6. Ang karbon mao ang wala puthaw.
Quick Facts Carbon, Panagway ...
| Carbon |
|---|
6C |
|
|
| Panagway |
clear (diamond) & black (graphite)


Spectral lines of Carbon |
| Kinatibuk-ang mga kinaiya |
| Ngalan, simbolo, kaiphan |
carbon, C, 6 |
| Paglitok |
|
| Kategoriyang elemento |
wala puthaw |
| Group, period, block |
14, 2, p |
| Gibug-aton sa atomo |
12.011(1) |
| Kontorno sa elektron |
[He] 2s2 2p2
2, 4
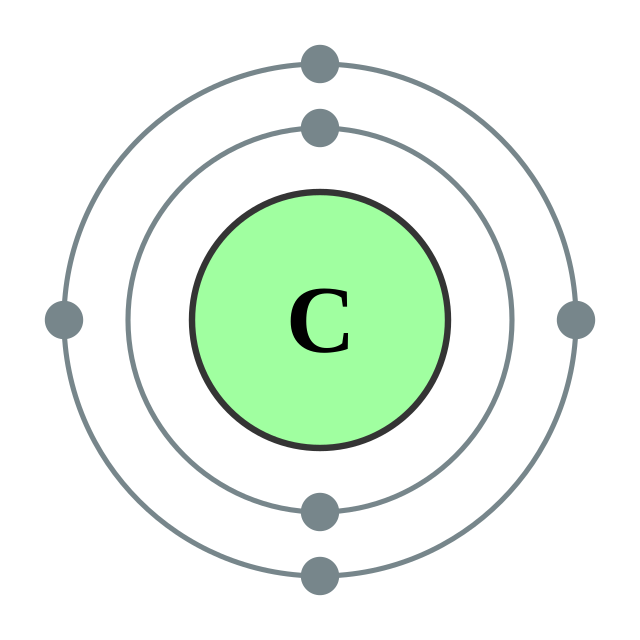 Electron shells of carbon (2, 4) Electron shells of carbon (2, 4) |
| History |
| Pagkadiskobre |
Egyptians and Sumerians[1] (3750 BC) |
| Recognized as an element by |
Antoine Lavoisier[2] (1789) |
| Physical properties |
| Phase |
magahi |
| Density (near r.t.) |
amorphous:[3] 1.8–2.1 g·cm−3 |
| Density (near r.t.) |
diamond: 3.515 g·cm−3 |
| Density (near r.t.) |
graphite: 2.267 g·cm−3 |
| Sublimation point |
3915 K, 3642 °C, 6588 °F |
| Triple point |
4600 K (4327°C), 10800[4][5] kPa |
| Heat of fusion |
117 (graphite) kJ·mol−1 |
| Molar heat capacity |
6.155 (diamond)
8.517 (graphite) J·mol−1·K−1 |
| Atomic properties |
| Oxidation states |
4, 3[6], 2, 1[7], 0, −1, −2, −3, −4[8] |
| Electronegativity |
2.55 (Pauling scale) |
Ionization energies
(more) |
1st: 1086.5 kJ·mol−1 |
| 2nd: 2352.6 kJ·mol−1 |
| 3rd: 4620.5 kJ·mol−1 |
| Covalent radius |
77(sp³), 73(sp²), 69(sp) pm |
| Van der Waals radius |
170 pm |
| Miscellanea |
| Crystal structure |
diamond
(diamond, clear) |
|
simple hexagonal
(graphite, black) |
| Magnetic ordering |
diamagnetic[9] |
| Thermal conductivity |
900-2300 (diamond)
119-165 (graphite) W·m−1·K−1 |
| Thermal expansion |
(25 °C) 0.8 (diamond)[10] µm·m−1·K−1 |
| Speed of sound (thin rod) |
(20 °C) 18350 (diamond) m·s−1 |
| Young's modulus |
1050 (diamond)[10] GPa |
| Shear modulus |
478 (diamond)[10] GPa |
| Bulk modulus |
442 (diamond)[10] GPa |
| Poisson ratio |
0.1 (diamond)[10] |
| Mohs hardness |
10 (diamond)
1-2 (graphite) |
| CAS registry number |
7440-44-0 |
| Most stable isotopes |
| Main article: Isotopes of carbon |
| iso |
NA |
half-life |
DM |
DE (MeV) |
DP
15 |
|---|
| 11C |
syn |
20 min |
β+ |
0.96 |
11B |
| 12C |
98.9% |
12C is stable with 6 neutrons |
| 13C |
1.1% |
13C is stable with 7 neutrons |
| 14C |
trace |
5730 y |
β− |
0.15 |
14N |
|
| · r |
Close



