Ang radon mao ang elementong kimiko sa talaang peryodiko nga may simbolo nga Rn ug kaiphan nga atomik 86. Ang radon mao ang gas nga halangdon.
Quick facts Panagway, Kinatibuk-ang mga kinaiya ...
| Radon |
|---|
86Rn |
|
|
| Panagway |
| colorless gas |
| Kinatibuk-ang mga kinaiya |
| Ngalan, simbolo, kaiphan |
radon, Rn, 86 |
| Paglitok |
[[Help:Pronunciation respelling key|Plantilya:Smallcaps all-don]] |
| Kategoriyang elemento |
gas nga halangdon |
| Group, period, block |
18, 6, p |
| Gibug-aton sa atomo |
(222) |
| Kontorno sa elektron |
[Xe] 4f14 5d10 6s2 6p6
2, 8, 18, 32, 18, 8
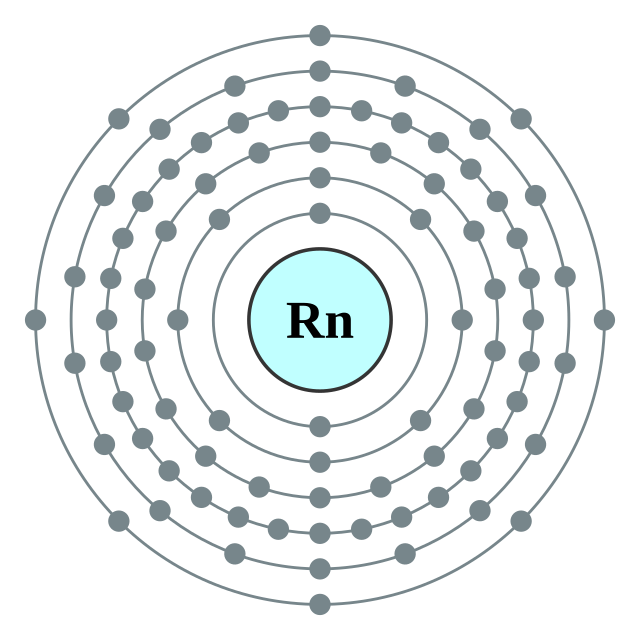 Electron shells of radon (2, 8, 18, 32, 18, 8) Electron shells of radon (2, 8, 18, 32, 18, 8) |
| History |
| Pagkadiskobre |
Friedrich Ernst Dorn (1898) |
| First isolation |
William Ramsay and Robert Whytlaw-Gray (1910) |
| Physical properties |
| Phase |
gas |
| Density |
(0 °C, 101.325 kPa)
9.73 g/L |
| Liquid density at b.p. |
4.4 g·cm−3 |
| Melting point |
202.0 K, −71.15 °C, −96.07 °F |
| Boiling point |
211.3 K, −61.85 °C, −79.1 °F |
| Critical point |
377 K, 6.28 MPa |
| Heat of fusion |
3.247 kJ·mol−1 |
| Heat of vaporization |
18.10 kJ·mol−1 |
| Molar heat capacity |
5R/2 = 20.786 J·mol−1·K−1 |
| Vapor pressure |
| P (Pa) |
1 |
10 |
100 |
1 k |
10 k |
100 k |
| at T (K) |
110 |
121 |
134 |
152 |
176 |
211 |
|
| Atomic properties |
| Oxidation states |
6, 2, 0 |
| Electronegativity |
2.2 (Pauling scale) |
| Ionization energies |
1st: 1037 kJ·mol−1 |
| Covalent radius |
150 pm |
| Van der Waals radius |
220 pm |
| Miscellanea |
| Crystal structure |
face-centered cubic
|
| Magnetic ordering |
non-magnetic |
| Thermal conductivity |
3.61 m W·m−1·K−1 |
| CAS registry number |
10043-92-2 |
| Most stable isotopes |
| Main article: Isotopes of radon |
| iso |
NA |
half-life |
DM |
DE (MeV) |
DP |
|---|
| 210Rn |
syn |
2.4 h |
α |
6.404 |
206Po |
| 211Rn |
syn |
14.6 h |
ε |
2.892 |
211At |
| α |
5.965 |
207Po |
| 222Rn |
trace |
3.8235 d |
α |
5.590 |
218Po |
| 224Rn |
syn |
1.8 h |
β− |
0.8 |
224Fr |
|
| · r |
Close


