Top Qs
Timeline
Chat
Perspective
Monoamine releasing agent
Class of compounds From Wikipedia, the free encyclopedia
Remove ads
A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of one or more monoamine neurotransmitters from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitters and hence enhanced signaling by those neurotransmitters.[2][3][4][1][5] The monoamine neurotransmitters include serotonin, norepinephrine, and dopamine; MRAs can induce the release of one or more of these neurotransmitters.[2][3][4][1][5]

MRAs work by reversing the direction of the monoamine transporters (MATs), including the serotonin transporter (SERT), norepinephrine transporter (NET), and/or dopamine transporter (DAT), causing them to promote efflux of non-vesicular cytoplasmic monoamine neurotransmitter rather than reuptake of synaptic monoamine neurotransmitter.[5][6][1][7] Many, but not all MRAs, also reverse the direction of the vesicular monoamine transporter 2 (VMAT2), thereby additionally resulting in efflux of vesicular monoamine neurotransmitter into the cytoplasm.[5]
A variety of different classes of drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters.[2][3] These include psychostimulants and appetite suppressants acting as dopamine and norepinephrine releasers like amphetamine, methamphetamine, and phentermine; sympathomimetic agents acting as norepinephrine releasers like ephedrine and pseudoephedrine; non-stimulant appetite suppressants acting as serotonin releasers like fenfluramine and chlorphentermine; and entactogens acting as releasers of serotonin and/or other monoamines like MDMA.[2][3] Trace amines like phenethylamine and tryptamine, as well as the monoamine neurotransmitters themselves, are endogenous MRAs.[2][3][4] It is thought that monoamine release by endogenous mediators may play some physiological regulatory role.[4]
MRAs must be distinguished from monoamine reuptake inhibitors (MRIs) and monoaminergic activity enhancers (MAEs), which similarly increase synaptic monoamine neurotransmitter levels and enhance monoaminergic signaling but work via distinct mechanisms.[5][1][8][9]
Remove ads
Types and selectivity
Summarize
Perspective
MRAs can be classified by the monoamines they mainly release, although these drugs lie on a spectrum:[2][3][4][5]
- Selective for one neurotransmitter
- Norepinephrine releasing agent (NRA) (e.g., ephedrine, levomethamphetamine)
- Dopamine releasing agent (DRA) (no robustly selective agents known)
- Serotonin releasing agent (SRA) (e.g., chlorphentermine, MMAI)
- Non-selective, releasing two or more neurotransmitters
- Norepinephrine–dopamine releasing agent (NDRA) (e.g., amphetamine, methamphetamine)
- Serotonin–norepinephrine releasing agent (SNRA) (e.g., fenfluramine, MDAI)
- Serotonin–dopamine releasing agent (SDRA) (e.g., 5-chloro-αMT, BK-NM-AMT)
- Serotonin–norepinephrine–dopamine releasing agent (SNDRA) (e.g., MDMA, mephedrone)
The differences in selectivity of MRAs is the result of different affinities as substrates for the monoamine transporters, and thus differing ability to gain access into monoaminergic neurons and induce monoamine neurotransmitter release.
As of present, no selective DRAs are known. This is because it has proven extremely difficult to separate DAT affinity from NET affinity and retain releasing efficacy at the same time.[10] Several selective SDRAs, including tryptamine, (+)-α-ethyltryptamine (αET), 5-chloro-αMT, and 5-fluoro-αET, are known.[11][12] However, besides their serotonin release, many of these compounds additionally act as non-selective serotonin receptor agonists, including of the serotonin 5-HT2A receptor (with accompanying hallucinogenic effects), and some of them are known to act as monoamine oxidase inhibitors.[11][12]
Remove ads
Effects and uses
Summarize
Perspective
MRAs can produce varying effects depending on their selectivity for inducing the release of different monoamine neurotransmitters.[3]
Selective SRAs such as chlorphentermine have been described as dysphoric and lethargic.[13][14] Less selective SRAs that also stimulate the release of dopamine, such as methylenedioxymethamphetamine (MDMA), are described as more pleasant, more reliably elevating mood and increasing energy and sociability.[15] SRAs have been used as appetite suppressants and as entactogens. They have also been proposed for use as more effective antidepressants and anxiolytics than selective serotonin reuptake inhibitors (SSRIs) because they can produce much larger increases in serotonin levels in comparison.[16]
DRAs, usually non-selective for both norepinephrine and dopamine, have psychostimulant effects, causing an increase in energy, motivation, elevated mood, and euphoria.[17] Other variables can significantly affect the subjective effects, such as infusion rate (increasing positive effects of DRAs) and psychological expectancy effects.[18] They are used in the treatment of attention deficit hyperactivity disorder (ADHD), as appetite suppressants, wakefulness-promoting agents, to improve motivation, and are drugs of recreational use and misuse.
Selective NRAs are minimally psychoactive, but as demonstrated by ephedrine, may be distinguished from placebo, and may trends towards liking.[19] They may also be performance-enhancing,[20] in contrast to reboxetine which is solely a norepinephrine reuptake inhibitor.[21][22] In addition to their central effects, NRAs produce peripheral sympathomimetic effects like increased heart rate, blood pressure, and force of heart contractions. They are used as nasal decongestants and bronchodilators, but have also seen use as wakefulness-promoting agents, appetite suppressants, and antihypotensive agents. They have additionally seen use as performance-enhancing drugs, for instance in sports.
Remove ads
Mechanism of action
Summarize
Perspective
Mechanisms of monoamine release by MRAs
MRAs induce the release of the monoamine neurotransmitters serotonin, norepinephrine, and/or dopamine from monoaminergic neurons in the brain and/or periphery.[3][23][24] MRAs are substrates of the plasma membrane-associated monoamine transporters (MATs), including of the serotonin transporter (SERT), norepinephrine transporter (NET), and/or dopamine transporter (DAT), and enter presynaptic monoaminergic neurons via these transporters.[23][3][24][25] To a much lesser extent, sufficiently lipophilic MRAs may also passively diffuse into monoaminergic neurons.[23][24] Once in the intracellular space of the neuron, MRAs reverse the direction of the MATs, as well as of the organic cation transporter 3 (OCT3),[23][26] such that they mediate efflux of cytosolic monoamine neurotransmitters into the extracellular synaptic cleft rather than the usual reuptake.[23][24] Many, though notably not all MRAs,[5][27][note 1] additionally act at the vesicular monoamine transporter 2 (VMAT2) on synaptic vesicles to enhance the pool of cytosolic monoamine neurotransmitters available for efflux.[23][5][31][24] However, MRAs can still induce monoamine release without VMAT2, for instance by releasing newly synthesized cytosolic neurotransmitters.[23][32][33] In addition to their induction of monoamine release, MRAs act less potently as monoamine reuptake inhibitors (MRIs).[23][24][2][1] This is due to substrate competition with monoamine neurotransmitters for the MATs[25][6][1] and/or induction of MAT internalization and consequent inactivation.[23][34] The monoamine neurotransmitters released by MRAs bind to and activate monoamine receptors on presynaptic and postsynaptic neurons to facilitate monoaminergic neurotransmission.[25][35] As such, MRAs can be described as indirect monoamine receptor agonists.[35][1]
The mechanisms by which MRAs induce MAT reverse transport and efflux are complex and incompletely understood.[23][24][36][37] The process appears to depend on a number of intracellular changes, including sodium ion (Na+) and calcium ion (Ca2+) elevation, protein kinase C (PKC) activation, and Ca2+/calmodulin-dependent protein kinase II alpha (CaMKIIα) activation, among others.[24][23][36][37][38] Activation of protein kinases including PKC, CaMKIIα, and others results in phosphorylation of the MATs causing them to mediate efflux instead of reuptake.[23][36][39][34] Exactly how MRAs induce the preceding effects is unclear however.[23][24][36][34][37] A more recent study suggests that intracellular Ca2+ elevation, PKC activation, and CaMKIIα might all be dispensable for MRA-induced monoamine release, but more research is needed.[40]
The trace amine-associated receptor 1 (TAAR1) is a receptor for trace amines like β-phenethylamine and tryptamine, as well as for monoamine neurotransmitters like dopamine and serotonin, and is a known target of many MRAs, such as amphetamine and methamphetamine.[41] The TAAR1 is a largely intracellular receptor expressed both in presynaptic and postsynaptic monoaminergic neurons and appears to be extensively co-localized with MATs in the brain.[42][41] Some in-vitro studies have found that TAAR1 agonism by MAT substrates like MRAs can produce PKC activation and thereby induce MAT reverse transport and monoamine efflux.[42][43] As such, TAAR1 agonism, coupled with MAT substrate activity, could mediate or contribute to the monoamine release of MRAs.[42][43] However, findings in this area are conflicting, with other studies unable to replicate the results.[44][45][46][47][48][49] In addition, MRAs can still induce monoamine efflux in the absence of TAAR1 in vitro,[50][51][42] well-known MRAs like amphetamine and methamphetamine exhibit only low-potency human TAAR1 agonism[52][53][35] that is of uncertain general significance in humans,[26][54][55][56][57] many other MRAs are inactive as TAAR1 agonists in humans,[52][53][25][26][note 2] the monoamine release and behavioral effects of amphetamines are not only preserved but substantially augmented in TAAR1 knockout mice,[44][42] and the monoamine release and behavioral effects of amphetamines are strongly reduced or abolished in mice with TAAR1 overexpression.[44][59] Besides induction of monoamine release, TAAR1 agonism, as well as other mechanisms, may mediate MAT internalization.[23][60] MAT internalization may limit the capacity of MRAs to induce MAT reverse transport and monoamine efflux.[61][62] TAAR1 signaling also activates G protein-coupled inwardly rectifying potassium channels (GIRKs) and thereby robustly inhibits the firing rates of brain monoaminergic neurons and suppresses exocytotic monoamine release.[63][42][46] Due to the preceding mechanisms, potent TAAR1 agonism by MRAs that possess this action may actually auto-inhibit and constrain their monoaminergic effects.[56][26][64][49]
Although induction of MAT reverse transport and consequent monoamine efflux is the leading theory of how MRAs act, an alternative and more recent theory has proposed that amphetamine, at therapeutic doses, may not actually act by inducing DAT reverse transport and dopamine efflux, but instead by augmenting exocytotic dopamine release and hence by enhancing phasic rather than tonic dopaminergic signaling.[23][65][66] According to this model, DAT reverse transport may only be relevant at supratherapeutic doses and may be more associated with toxicity, for instance induction of psychosis.[23][65][66] It is unclear how amphetamine might act to enhance exocytotic dopamine release, and more research is needed to evaluate this theory.[23][65][66]
Aside from the mechanisms mediating the monoamine release of MRAs, other targets of some MRAs, such as the intracellular sigma σ1 receptor, have been found to inhibit MRA-induced monoamine efflux via interactions with the MATs.[67][68] Conversely, activation of the sigma σ2 receptor has been found to potentiate amphetamine-induced dopamine efflux.[69] The mechanism mediating this effect is unknown, but it has been postulated that it may be due to elevation of intracellular calcium and consequent downstream effects.[69]
Differences from physiological release and reuptake inhibitors
The neurotransmitter release induced by MRAs is very different from normal exocytotic monoamine release, in which action potentials trigger synaptic vesicles to fuse with the cell membrane and release neurotransmitters into the synaptic cleft.[23][3] In relation to this, MRAs promote tonic monoaminergic signaling, whereas normal exocytotic monoamine release involves phasic monoaminergic signaling.[23]
The enhancement of monoaminergic signaling by MRAs also differs from that with MRIs.[3][5][23][36] Because MRIs block monoamine neurotransmitter reuptake and consequent inactivation following action potentials and exocytotic release, they preferentially augment phasic monoaminergic signaling rather than tonic signaling.[3] In addition, inhibitory presynaptic and somatodendritic monoamine autoreceptors, including serotonin 5-HT1A and 5-HT1B autoreceptors, dopamine D2 and D3 autoreceptors, and α2-adrenergic autoreceptors, respond to elevated synaptic monoamine neurotransmitter levels by inhibiting presynaptic monoaminergic neuron firing rates, and this substantially limits the effects of MRIs.[3][5][35] In contrast, MRAs do not depend on action potentials to induce monoamine release, and thus are able to largely bypass the negative feedback mediated by autoreceptors.[3] Relatedly, MRAs can induce far greater maximal increases in monoamine neurotransmitter levels than MRIs.[3] For instance, MRIs can achieve maximal elevations in brain monoamine levels of about 5- to 10-fold in animals, whereas MRAs can produce elevations of as much as 10- to 50-fold, with no clear ceiling limit.[1][70][71][72][73][3][74] Since MRAs depend on uptake by the MATs to induce monoamine release, their mediation of monoamine release and consequent effects can be blocked by MRIs.[3][23][24][75]
Structural requirements and partial MRAs

There is a constrained and relatively small molecular size requirement for compounds to act as MRAs.[5] This is because they must be small enough to serve as substrates of the monoamine transporters and thereby be transported inside of monoaminergic neurons by these proteins, in turn allowing them to induce monoamine neurotransmitter release.[5][23] Compounds with chemical features extending beyond the size constraints for releasers will instead act as partial releasers, reuptake inhibitors, or be inactive.[5][23] Partial releasers show reduced maximal efficacy in releasing monoamine neurotransmitters compared to conventional full releasers.[5][6][23] While most MRAs are full releasers, a number of partial releasers are known and may have atypical properties.[5][6] Examples of partial releasers include 3,4-methylenedioxyethylamphetamine (MDEA), N-ethylnaphthylaminopropane (ENAP), 3-trifluoromethyl-4-chlorophenylpiperazine (TFMCPP), para-nitrophenylpiperazine (pNPP), bretisilocin, and psilocin.[5][6][76][77][78] The mechanisms responsible for the differences between full releasers and partial releasers are largely unknown.[6]
Other related agents
DAT "inverse agonists"
Dopamine reuptake inhibitors (DRIs) have been grouped into two types, typical or conventional DRIs like cocaine, WIN-35428 (β-CFT), and methylphenidate that produce potent psychostimulant, euphoric, and reinforcing effects, and atypical DRIs like vanoxerine (GBR-12909), modafinil, benztropine, and bupropion, which do not produce such effects or have greatly reduced such effects.[7][6][5][79] It has been proposed that typical DRIs may not actually be acting primarily as DRIs but rather as dopamine releasing agents (DRAs) via mechanisms distinct from conventional substrate-type DRAs like amphetamines.[7] A variety of different pieces of evidence support this hypothesis and help to explain otherwise confusing findings.[7] For example, typical DRIs like cocaine and methylphenidate can robustly increase brain dopamine levels similarly to substrate-type DRAs like amphetamine, whereas atypical DRIs, which are viewed as simple competitive reuptake inhibitors, achieve much more modest increases.[7][80][81][82] Under this model, typical cocaine-like DRIs have been referred to with the new label of dopamine transporter (DAT) "inverse agonists" to distinguish them from conventional substrate-type DRAs.[7] An alternative theory is that typical DRIs and atypical DRIs stabilize the DAT in different conformations, with typical DRIs resulting in an outward-facing open conformation that produces differing pharmacological effects from those of atypical DRIs.[6][5][79][83]
Monoaminergic activity enhancers
Some MRAs, like the amphetamines amphetamine and methamphetamine, as well as trace amines like phenethylamine, tryptamine, and tyramine, are additionally monoaminergic activity enhancers (MAEs).[8][9][84] That is, they enhance the action potential-mediated release of monoamine neurotransmitters (in contrast to MRAs, which induce uncontrolled monoamine release independent of neuronal firing).[8][9][84] They are usually active as MAEs at much lower concentrations than those at which they induce monoamine release.[8][9][84] The MAE actions of MAEs may be mediated by TAAR1 agonism, which has likewise been implicated in monoamine-releasing actions in some studies.[85][86][87] MAEs without concomitant potent monoamine-releasing actions, like selegiline (L-deprenyl), phenylpropylaminopentane (PPAP), and benzofuranylpropylaminopentane (BPAP), have been developed.[8][9]
Remove ads
Endogenous MRAs
Summarize
Perspective
A number of endogenous compounds are known to act as MRAs.[4][88][77][11][5] These include the monoamine neurotransmitters dopamine (an NDRA),[88] norepinephrine (an NDRA),[88] and serotonin (an SRA) themselves,[88] as well as the trace amines phenethylamine (an NDRA),[5][84][89][90] tryptamine (an SDRA or imbalanced SNDRA),[77][11] and tyramine (an NDRA).[88][4] Synthetic MRAs are substantially based on structural modification of these endogenous compounds, most prominently including the substituted phenethylamines and substituted tryptamines.[88][2][3][77][91][92][93]
Release of monoamine neurotransmitters by themselves, for instance in the cases of serotonin, norepinephrine, and dopamine, has been referred to as "self-release".[4] The physiological significance of the findings that monoamine neurotransmitters can act as releasing agents of themselves is unclear.[4] However, it could imply that efflux is a common neurotransmitter regulatory mechanism that can be induced by any transporter substrate.[4]
It is possible that monoamine neurotransmitter self-release could be a protective mechanism.[4][94] It is notable in this regard that intracellular non-vesicular or cytoplasmic dopamine is toxic to neurons and that the vesicular monoamine transporter 2 (VMAT2) is neuroprotective by packaging this dopamine into synaptic vesicles.[95][96][97][94] Along similar lines, MRAs induce the efflux of non-vesicular monoamine neurotransmitter and thereby move cytoplasmic neurotransmitter into the extracellular space.[5] On the other hand, many MRAs but not all also act as VMAT2 inhibitors and reversers, and hence concomitantly induce the release of vesicular monoamine neurotransmitters like dopamine into the cytoplasm.[5] Induction of VMAT2 efflux by MRAs appears to be related to their monoaminergic neurotoxicity.[35][98][29]
Remove ads
Monoaminergic neurotoxicity
Some MRAs have been found to act as monoaminergic neurotoxins and hence to produce long-lasting damage to monoaminergic neurons.[99][100] Examples include dopaminergic neurotoxicity with amphetamine and methamphetamine and serotonergic neurotoxicity with methylenedioxymethamphetamine (MDMA).[99][100] Amphetamine may produce significant dopaminergic neurotoxicity even at therapeutic doses.[101][102][103][104][105][106] However, clinical doses of amphetamine producing neurotoxicity is controversial and disputed.[107][101][103] In contrast to amphetamines, monoamine reuptake inhibitors like methylphenidate lack apparent neurotoxic effects.[101]
Analogues of MDMA with retained MRA activity but reduced or no serotonergic neurotoxicity, like 5,6-methylenedioxy-2-aminoindane (MDAI) and 5-iodo-2-aminoindane (5-IAI), have been developed.[28][108] Certain drugs have been found to block the neurotoxicity of MRAs in animals.[100] For instance, the selective MAO-B inhibitor selegiline has been found to prevent the serotonergic neurotoxicity of MDMA in rodents.[100]
Remove ads
Chemical families
Summarize
Perspective
MRAs are usually arylalkylamines. A number of different structural families of compounds have been found to act as MRAs. The possible structural forms of MRAs are limited by a small molecular size requirement for activity.[5] Molecules that are too large become monoamine reuptake inhibitors as they can no longer be transported into neurons by the monoamine transporters and induce monoamine release intracellularly.[5]
Phenethylamine-like
- Phenethylamines (2-phenylethylamines) (e.g., phenethylamine, tyramine, dopamine, norepinephrine)
- Amphetamines (α-methylphenethylamines) (e.g., amphetamine, methamphetamine, fenfluramine)
- Cathinols (β-hydroxyamphetamines) (e.g., phenylpropanolamine, ephedrine, pseudoephedrine, cathine)
- Cathinones (β-ketoamphetamines) (e.g., cathinone, methcathinone, mephedrone)
- Phentermines (α-methylamphetamines) (e.g., phentermine, mephentermine, chlorphentermine, clortermine)
- Phenylisobutylamines (α-ethylphenethylamines) (e.g., phenylisobutylamine, 4-CAB, 4-MAB, buphedrone)
- Ring-extended amphetamines
- Benzodioxolylaminopropanes (methylenedioxyamphetamines) (e.g., MDA, MDMA, MDEA, methylone)
- Methylenedioxycathinones (e.g., methylone, ethylone, butylone)
- Methylenedioxyphentermines (e.g., MDPH, MDMPH)
- Benzodioxolylisobutylamines (e.g., BDB, MBDB, butylone)[109]
- Benzodioxanylaminopropanes (ethylenedioxyamphetamines) (e.g., EDA, EDMA, EDMC)[110][111][112]
- Benzofuranylaminopropanes (e.g., 4-APB, 5-APB, 5-MAPB, 6-APB, 6-MAPB, 5-APDB, 5-MAPDB, 6-APDB, 6-MAPDB, 7-APB, IBF5MAP)[113][114][115]
- Benzofuranylisobutylamines (e.g., 5-MBPB, 6-MBPB)[116]
- Benzothiophenylaminopropanes (e.g., 4-APBT, 5-APBT, 5-MAPBT, 6-APBT, 7-APBT)[117]
- Indolylaminopropanes (e.g., 5-IT/5-API, 6-IT/6-API)[118]
- Indanylaminopropanes (e.g., 5-APDI/IAP, 5-MAPDI)[115][119]
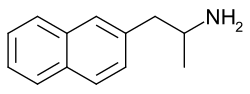
- Naphthylaminopropanes (e.g., NAP, methamnetamine/MNAP, ENAP, BMAPN)[76][120]
- Tetralinylaminopropanes (e.g., 6-APT/TAP)[115]
- Other ring-extended amphetamines (e.g., ODMA,[121] SeDMA,[121] TDMA[121])
- Benzodioxolylaminopropanes (methylenedioxyamphetamines) (e.g., MDA, MDMA, MDEA, methylone)
- Amphetamines (α-methylphenethylamines) (e.g., amphetamine, methamphetamine, fenfluramine)
- Cyclopentylaminopropanes (e.g., isocyclamine, cyclopentamine)[122]
- Cyclohexylaminopropanes (e.g., norpropylhexedrine, propylhexedrine)[122]
- Phenylpropylamines (e.g., phenylpropylamine, homo-MDA, homo-MDMA)[123][4][124][125]
- Thiophenylisopropylamines (e.g., thiopropamine, methiopropamine, thiothinone)[126]
- Phenylalkenylamines and phenylalkynylamines (e.g., phenylbutynamine, phenylbutenamine)[127][128][76]
- Other possible groups (e.g., 2-furylethylamines, 2-tetrahydrofurylethylamines, 2-pyrrolylethylamines, 3-pyrrolylethylamines)[93][126]
Amine fused into ring
- Phenylalkylpyrrolidines (e.g., α-PPP, 4-MePPP)[129][130]
- Phenylmorpholines (e.g., phenmetrazine, phendimetrazine)
- Phenyloxazolamines (e.g., aminorex, 4-methylaminorex, pemoline)
- Benzylpiperazines (e.g., 1-benzylpiperazine, MBZP)
- Methylenedioxybenzylpiperazines (e.g., MDBZP, fipexide)
- Benzylpiperidines (e.g., 4-benzylpiperidine)
- Phenylpiperazines (e.g., 1-phenylpiperazine, mCPP, TFMPP, oMPP, pFPP, pMeOPP)[131]
Alkyl chain fused into ring
- 2-Aminoindanes (e.g., 2-aminoindane (2-AI), NM-2-AI, MMAI, MEAI, ETAI, TAI, 5-IAI)[33][132][120]
- Methylenedioxyaminoindanes (e.g., MDAI, MDMAI)[33][132]
- Benzofurancyclopentanylamines (e.g., BFAI)[133]
- 2-Aminotetralins (e.g., 2-aminotetralin, 6-CAT)[132][120]
- Methylenedioxyaminotetralins (e.g., MDAT, MDMAT)[132]
- Other possible groups (e.g., benzylamines (e.g., MDM1EA),[125] 2-amino-1,2-dihydronaphthalenes (e.g., 2-ADN),[134][135] aminobenzocycloheptenes (e.g., 6-AB, 7-AB)[120][136])
Tryptamine-like
- Tryptamines (2-(3-indolyl)ethylamines) (e.g., tryptamine, serotonin, bufotenin, DMT, psilocin, bretisilocin)[77][11]
- α-Alkyltryptamines (e.g., αMT (3-API), αET, 5-chloro-αMT, 5-fluoro-αMT)[11][109]
- Isotryptamines (2-(1-indolyl)ethylamines) (e.g., isoAMT)[12]
- Indolizinylaminopropanes (e.g., 1ZP2MA, 1Z2MAP1O)[139][140]
- Benzothiophenylaminopropanes (e.g., 2-APBT, 3-APBT)[117]
- Benzofuranylaminopropanes (e.g., 2-APB, 2-MAPB, 3-APB)[114]
- Other possible groups (e.g., 2-pyrrolylethylamines, 3-pyrrolylethylamines)[93][126]
Ring-less (alkylamines)
Chemical family structures gallery
Chemical family structure examples of monoamine releasing agents
Remove ads
Activity profiles
Summarize
Perspective
The activities of many MRAs in terms of their potencies, efficacies, and selectivities for monoamine release induction in vitro have been characterized in numerous studies in the scientific literature.[2][71][3][5][149] These studies have been especially conducted by the research lab led by Richard B. Rothman and Michael H. Baumann at the National Institute on Drug Abuse (NIDA).[2][71][3][149] These researchers developed an assay measuring monoamine release from rat brain synaptosomes in 1999 that has subsequently been widely employed.[149][71][88][150][151] The data with this procedure from many relevant studies are provided in the table below.[2][3] The Rothman and Baumann lab refers to these data as the "Phenyl Amine Library", "Phenethylamine Library", "Phenylethylamine Library", or "PAL" library, a large library of values of phenethylamine analogs at the monoamine transporters (1,400 compounds as of 2015), and has designated PAL-# code names for the drugs included in it.[152][5][149]
Another method of measuring monoamine release involves the use of human HEK293 cells transfected with and expressing monoamine transporters.[51][58][28][153][110] However, MRAs show differing and much lower potencies in this system compared to rat brain synaptosomes, and it is much less frequently employed.[51][58][28][153][110] The reasons for these differences are not entirely clear, but may be related to species differences, differences in release assay methods, and/or absence of important neuronal membrane proteins in non-neuronal HEK293 cells.[110][50]
In addition to the potencies of MRAs in terms of their MRA activity, data on the affinities (Ki) of various MRAs for the monoamine transporters (MATs) and their potencies (IC50) in acting as monoamine reuptake inhibitors (MRIs) have been published.[2][88][224][225][206][190][212][188][172][175][226][227][116][158][219][163][117][228][131][156] Activities of MRAs at the vesicular monoamine transporter 2 (VMAT2) have been published as well.[27]
Remove ads
Notes
- MRAs that are inactive at VMAT2 include phentermine, phenmetrazine, and benzylpiperazine (BZP).[5][27] Others, including cathinones like mephedrone, methcathinone, and methylone, also show only weak VMAT2 activity (e.g., ~10-fold weaker than the corresponding amphetamines).[28][29][30]
- MRAs that are inactive at the human TAAR1 include most cathinones (e.g., methcathinone, mephedrone, flephedrone, and brephedrone), ephedrine, 4-methylamphetamine (4-MA), para-methoxyamphetamine (PMA), 4-methylthioamphetamine (4-MTA), MDMA MDEA, MBDB, 5-APDB, 5-MAPDB, 4-methylaminorex derivatives, meta-chlorophenylpiperazine (mCPP), TFMPP, and methylhexanamine (DMAA), among others.[52][53][26][50][58]
Remove ads
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads