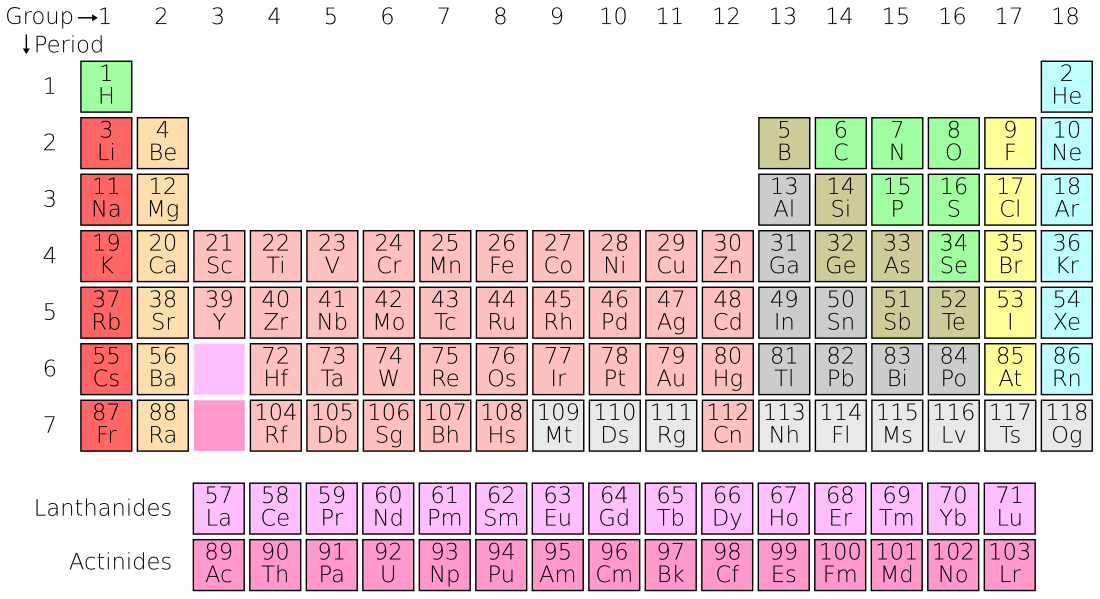
Z
|
Symbol
|
Element
|
Origin of name[5]
|
Group
|
Period
|
Weight
u ()
|
സാന്ദ്രത
g / cm3
|
ദ്രവണാങ്കം
K
|
ക്വഥനാങ്കം
K
|
താപധാരിത
J/g·K
|
വിദ്യുത് ഋണത10 |
Abundance
mg / kg
|
| −999 | !a | !a | −999 | −999 | −999 | −999 | −999 | −999 | −999 | −999 | −999 |
| 1 | H | Hydrogen | the Greek 'hydro' and 'genes' meaning water-forming | 1 | 1 | 1.008(1)2 3 4 9 | 0.00008988 | 14.01 | 20.28 | 14.304 | 2.20 | 1400 |
| 2 | He | Helium | the Greek, 'helios' meaning sun | 18 | 1 | 4.002602(2)2 4 | 0.0001785 | 0.956 | 4.22 | 5.193 | – | 0.008 |
| 3 | Li | Lithium | the Greek 'lithos' meaning stone | 1 | 2 | 6.94(1)2 3 4 5 9 | 0.534 | 453.69 | 1560 | 3.582 | 0.98 | 20 |
| 4 | Be | Beryllium | the Greek name for beryl, 'beryllo' | 2 | 2 | 9.012182(3) | 1.85 | 1560 | 2742 | 1.825 | 1.57 | 2.8 |
| 5 | B | Boron | the Arabic 'buraq', which was the name for borax | 13 | 2 | 10.81(1)2 3 4 9 | 2.34 | 2349 | 4200 | 1.026 | 2.04 | 10 |
| 6 | C | Carbon | the Latin 'carbo', meaning charcoal | 14 | 2 | 12.011(1)2 4 9 | 2.267 | 3800 | 4300 | 0.709 | 2.55 | 200 |
| 7 | N | Nitrogen | the Greek 'nitron' and 'genes' meaning nitre-forming | 15 | 2 | 14.007(1)2 4 9 | 0.0012506 | 63.15 | 77.36 | 1.04 | 3.04 | 19 |
| 8 | O | Oxygen | the Greek 'oxy' and 'genes' meaning acid-forming | 16 | 2 | 15.999(1)2 4 9 | 0.001429 | 54.36 | 90.20 | 0.918 | 3.44 | 461000 |
| 9 | F | Fluorine | the Latin 'fluere', meaning to flow | 17 | 2 | 18.9984032(5) | 0.001696 | 53.53 | 85.03 | 0.824 | 3.98 | 585 |
| 10 | Ne | Neon | the Greek 'neos', meaning new | 18 | 2 | 20.1797(6)2 3 | 0.0008999 | 24.56 | 27.07 | 1.03 | – | 0.005 |
| 11 | Na | Sodium | the English word soda (natrium in Latin)[6] | 1 | 3 | 22.98976928(2) | 0.971 | 370.87 | 1156 | 1.228 | 0.93 | 23600 |
| 12 | Mg | Magnesium | Magnesia, a region in Greece | 2 | 3 | 24.3050(6) | 1.738 | 923 | 1363 | 1.023 | 1.31 | 23300 |
| 13 | Al | Aluminium | the Latin name for alum, 'alumen' meaning bitter salt | 13 | 3 | 26.9815386(8) | 2.698 | 933.47 | 2792 | 0.897 | 1.61 | 82300 |
| 14 | Si | Silicon | the Latin 'silex' or 'silicis', meaning flint | 14 | 3 | 28.085(1)4 9 | 2.3296 | 1687 | 3538 | 0.705 | 1.9 | 282000 |
| 15 | P | Phosphorus | the Greek 'phosphoros', meaning bringer of light | 15 | 3 | 30.973762(2) | 1.82 | 317.30 | 550 | 0.769 | 2.19 | 1050 |
| 16 | S | Sulfur | Either from the Sanskrit 'sulvere', or the Latin 'sulfurium', both names for sulfur[6] | 16 | 3 | 32.06(1)2 4 9 | 2.067 | 388.36 | 717.87 | 0.71 | 2.58 | 350 |
| 17 | Cl | Chlorine | the Greek 'chloros', meaning greenish yellow | 17 | 3 | 35.45(1)2 3 4 9 | 0.003214 | 171.6 | 239.11 | 0.479 | 3.16 | 145 |
| 18 | Ar | Argon | the Greek, 'argos', meaning idle | 18 | 3 | 39.948(1)2 4 | 0.0017837 | 83.80 | 87.30 | 0.52 | – | 3.5 |
| 19 | K | Potassium | the English word potash (kalium in Latin)[6] | 1 | 4 | 39.0983(1) | 0.862 | 336.53 | 1032 | 0.757 | 0.82 | 20900 |
| 20 | Ca | Calcium | the Latin 'calx' meaning lime | 2 | 4 | 40.078(4)2 | 1.54 | 1115 | 1757 | 0.647 | 1 | 41500 |
| 21 | Sc | Scandium | Scandinavia (with the Latin name Scandia) | 3 | 4 | 44.955912(6) | 2.989 | 1814 | 3109 | 0.568 | 1.36 | 22 |
| 22 | Ti | Titanium | Titans, the sons of the Earth goddess of Greek mythology | 4 | 4 | 47.867(1) | 4.54 | 1941 | 3560 | 0.523 | 1.54 | 5650 |
| 23 | V | Vanadium | Vanadis, an old Norse name for the goddess Freyja | 5 | 4 | 50.9415(1) | 6.11 | 2183 | 3680 | 0.489 | 1.63 | 120 |
| 24 | Cr | Chromium | the Greek 'chroma', meaning colour | 6 | 4 | 51.9961(6) | 7.15 | 2180 | 2944 | 0.449 | 1.66 | 102 |
| 25 | Mn | Manganese | Either the Latin 'magnes', meaning magnet or from the black magnesium oxide, 'magnesia nigra' | 7 | 4 | 54.938045(5) | 7.44 | 1519 | 2334 | 0.479 | 1.55 | 950 |
| 26 | Fe | Iron | the Anglo-Saxon name iren (ferrum in Latin) | 8 | 4 | 55.845(2) | 7.874 | 1811 | 3134 | 0.449 | 1.83 | 56300 |
| 27 | Co | Cobalt | the German word 'kobald', meaning goblin | 9 | 4 | 58.933195(5) | 8.86 | 1768 | 3200 | 0.421 | 1.88 | 25 |
| 28 | Ni | Nickel | the shortened of the German 'kupfernickel' meaning either devil's copper or St. Nicholas's copper | 10 | 4 | 58.6934(4) | 8.912 | 1728 | 3186 | 0.444 | 1.91 | 84 |
| 29 | Cu | Copper | the Old English name coper in turn derived from the Latin 'Cyprium aes', meaning a metal from Cyprus | 11 | 4 | 63.546(3)4 | 8.96 | 1357.77 | 2835 | 0.385 | 1.9 | 60 |
| 30 | Zn | Zinc | the German, 'zinc', which may in turn be derived from the Persian word 'sing', meaning stone | 12 | 4 | 65.38(2) | 7.134 | 692.88 | 1180 | 0.388 | 1.65 | 70 |
| 31 | Ga | Gallium | France (with the Latin name Gallia) | 13 | 4 | 69.723(1) | 5.907 | 302.9146 | 2477 | 0.371 | 1.81 | 19 |
| 32 | Ge | Germanium | Germany (with the Latin name Germania) | 14 | 4 | 72.63(1) | 5.323 | 1211.40 | 3106 | 0.32 | 2.01 | 1.5 |
| 33 | As | Arsenic | the Greek name 'arsenikon' for the yellow pigment orpiment | 15 | 4 | 74.92160(2) | 5.776 | 1090 7 | 887 | 0.329 | 2.18 | 1.8 |
| 34 | Se | Selenium | Moon (with the Greek name selene) | 16 | 4 | 78.96(3)4 | 4.809 | 453 | 958 | 0.321 | 2.55 | 0.05 |
| 35 | Br | Bromine | the Greek 'bromos' meaning stench | 17 | 4 | 79.904(1) | 3.122 | 265.8 | 332.0 | 0.474 | 2.96 | 2.4 |
| 36 | Kr | Krypton | the Greek 'kryptos', meaning hidden | 18 | 4 | 83.798(2)2 3 | 0.003733 | 115.79 | 119.93 | 0.248 | 3 | <0.001 |
| 37 | Rb | Rubidium | the Latin 'rubidius', meaning deepest red | 1 | 5 | 85.4678(3)2 | 1.532 | 312.46 | 961 | 0.363 | 0.82 | 90 |
| 38 | Sr | Strontium | Strontian, a small town in Scotland | 2 | 5 | 87.62(1)2 4 | 2.64 | 1050 | 1655 | 0.301 | 0.95 | 370 |
| 39 | Y | Yttrium | Ytterby, Sweden | 3 | 5 | 88.90585(2) | 4.469 | 1799 | 3609 | 0.298 | 1.22 | 33 |
| 40 | Zr | Zirconium | the Persian 'zargun', meaning gold coloured | 4 | 5 | 91.224(2)2 | 6.506 | 2128 | 4682 | 0.278 | 1.33 | 165 |
| 41 | Nb | Niobium | Niobe, daughter of king Tantalus from Greek mythology | 5 | 5 | 92.90638(2) | 8.57 | 2750 | 5017 | 0.265 | 1.6 | 20 |
| 42 | Mo | Molybdenum | the Greek 'molybdos' meaning lead | 6 | 5 | 95.96(2)2 | 10.22 | 2896 | 4912 | 0.251 | 2.16 | 1.2 |
| 43 | Tc | Technetium | the Greek 'tekhnetos' meaning artificial | 7 | 5 | [98]1 | 11.5 | 2430 | 4538 | – | 1.9 | <0.001 |
| 44 | Ru | Ruthenium | Russia (with the Latin name Ruthenia) | 8 | 5 | 101.07(2)2 | 12.37 | 2607 | 4423 | 0.238 | 2.2 | 0.001 |
| 45 | Rh | Rhodium | the Greek 'rhodon', meaning rose coloured | 9 | 5 | 102.90550(2) | 12.41 | 2237 | 3968 | 0.243 | 2.28 | 0.001 |
| 46 | Pd | Palladium | From the asteroid Pallas which had been recently discovered and named at the time. The asteroid was thought to be a planet when it was discovered | 10 | 5 | 106.42(1)2 | 12.02 | 1828.05 | 3236 | 0.244 | 2.2 | 0.015 |
| 47 | Ag | Silver | the Anglo-Saxon name siolfur (argentum in Latin)[6] | 11 | 5 | 107.8682(2)2 | 10.501 | 1234.93 | 2435 | 0.235 | 1.93 | 0.075 |
| 48 | Cd | Cadmium | the Latin name for the mineral calmine, 'cadmia' | 12 | 5 | 112.411(8)2 | 8.69 | 594.22 | 1040 | 0.232 | 1.69 | 0.159 |
| 49 | In | Indium | the Latin 'indicium', meaning violet or indigo | 13 | 5 | 114.818(3) | 7.31 | 429.75 | 2345 | 0.233 | 1.78 | 0.25 |
| 50 | Sn | Tin | the Anglo-Saxon word tin (stannum in Latin, meaning hard) | 14 | 5 | 118.710(7)2 | 7.287 | 505.08 | 2875 | 0.228 | 1.96 | 2.3 |
| 51 | Sb | Antimony | the Greek 'anti – monos', meaning not alone (stibium in Latin) | 15 | 5 | 121.760(1)2 | 6.685 | 903.78 | 1860 | 0.207 | 2.05 | 0.2 |
| 52 | Te | Tellurium | Earth, the third planet on solar system (with the Latin word tellus) | 16 | 5 | 127.60(3)2 | 6.232 | 722.66 | 1261 | 0.202 | 2.1 | 0.001 |
| 53 | I | Iodine | the Greek 'iodes' meaning violet | 17 | 5 | 126.90447(3) | 4.93 | 386.85 | 457.4 | 0.214 | 2.66 | 0.45 |
| 54 | Xe | Xenon | the Greek 'xenos' meaning stranger | 18 | 5 | 131.293(6)2 3 | 0.005887 | 161.4 | 165.03 | 0.158 | 2.6 | <0.001 |
| 55 | Cs | Caesium | the Latin 'caesius', meaning sky blue | 1 | 6 | 132.9054519(2) | 1.873 | 301.59 | 944 | 0.242 | 0.79 | 3 |
| 56 | Ba | Barium | the Greek 'barys', meaning heavy | 2 | 6 | 137.327(7) | 3.594 | 1000 | 2170 | 0.204 | 0.89 | 425 |
| 57 | La | Lanthanum | the Greek 'lanthanein', meaning to lie hidden | | 6 | 138.90547(7)2 | 6.145 | 1193 | 3737 | 0.195 | 1.1 | 39 |
| 58 | Ce | Cerium | Ceres, the Roman God of agriculture | | 6 | 140.116(1)2 | 6.77 | 1068 | 3716 | 0.192 | 1.12 | 66.5 |
| 59 | Pr | Praseodymium | the Greek 'prasios didymos' meaning green twin | | 6 | 140.90765(2) | 6.773 | 1208 | 3793 | 0.193 | 1.13 | 9.2 |
| 60 | Nd | Neodymium | the Greek 'neos didymos' meaning new twin | | 6 | 144.242(3)2 | 7.007 | 1297 | 3347 | 0.19 | 1.14 | 41.5 |
| 61 | Pm | Promethium | Prometheus of Greek mythology who stole fire from the Gods and gave it to humans | | 6 | [145]1 | 7.26 | 1315 | 3273 | – | – | <0.001 |
| 62 | Sm | Samarium | Samarskite, the name of the mineral from which it was first isolated | | 6 | 150.36(2)2 | 7.52 | 1345 | 2067 | 0.197 | 1.17 | 7.05 |
| 63 | Eu | Europium | Europe | | 6 | 151.964(1)2 | 5.243 | 1099 | 1802 | 0.182 | 1.2 | 2 |
| 64 | Gd | Gadolinium | Johan Gadolin, chemist, physicist and mineralogist | | 6 | 157.25(3)2 | 7.895 | 1585 | 3546 | 0.236 | 1.2 | 6.2 |
| 65 | Tb | Terbium | Ytterby, Sweden | | 6 | 158.92535(2) | 8.229 | 1629 | 3503 | 0.182 | 1.2 | 1.2 |
| 66 | Dy | Dysprosium | the Greek 'dysprositos', meaning hard to get | | 6 | 162.500(1)2 | 8.55 | 1680 | 2840 | 0.17 | 1.22 | 5.2 |
| 67 | Ho | Holmium | Stockholm, Sweden (with the Latin name Holmia) | | 6 | 164.93032(2) | 8.795 | 1734 | 2993 | 0.165 | 1.23 | 1.3 |
| 68 | Er | Erbium | Ytterby, Sweden | | 6 | 167.259(3)2 | 9.066 | 1802 | 3141 | 0.168 | 1.24 | 3.5 |
| 69 | Tm | Thulium | Thule, the ancient name for Scandinavia | | 6 | 168.93421(2) | 9.321 | 1818 | 2223 | 0.16 | 1.25 | 0.52 |
| 70 | Yb | Ytterbium | Ytterby, Sweden | | 6 | 173.054(5)2 | 6.965 | 1097 | 1469 | 0.155 | 1.1 | 3.2 |
| 71 | Lu | Lutetium | Paris, France (with the Roman name Lutetia) | 3 | 6 | 174.9668(1)2 | 9.84 | 1925 | 3675 | 0.154 | 1.27 | 0.8 |
| 72 | Hf | Hafnium | Copenhagen, Denmark (with the Latin name Hafnia) | 4 | 6 | 178.49(2) | 13.31 | 2506 | 4876 | 0.144 | 1.3 | 3 |
| 73 | Ta | Tantalum | King Tantalus, father of Niobe from Greek mythology | 5 | 6 | 180.94788(2) | 16.654 | 3290 | 5731 | 0.14 | 1.5 | 2 |
| 74 | W | Tungsten | the Swedish 'tung sten' meaning heavy stone (W is wolfram, the old name of the tungsten mineral wolframite)[6] | 6 | 6 | 183.84(1) | 19.25 | 3695 | 5828 | 0.132 | 2.36 | 1.3 |
| 75 | Re | Rhenium | Rhine River, in Europe (with the Latin name Rhenia) | 7 | 6 | 186.207(1) | 21.02 | 3459 | 5869 | 0.137 | 1.9 | <0.001 |
| 76 | Os | Osmium | the Greek 'osme', meaning smell | 8 | 6 | 190.23(3)2 | 22.61 | 3306 | 5285 | 0.13 | 2.2 | 0.002 |
| 77 | Ir | Iridium | Iris, the Greek goddess of the rainbow | 9 | 6 | 192.217(3) | 22.56 | 2719 | 4701 | 0.131 | 2.2 | 0.001 |
| 78 | Pt | Platinum | the Spanish 'platina', meaning little silver | 10 | 6 | 195.084(9) | 21.46 | 2041.4 | 4098 | 0.133 | 2.28 | 0.005 |
| 79 | Au | Gold | the Anglo-Saxon word gold (aurum in Latin, meaning glow of sunrise)[6] | 11 | 6 | 196.966569(4) | 19.282 | 1337.33 | 3129 | 0.129 | 2.54 | 0.004 |
| 80 | Hg | Mercury | Mercury, the first planet in the Solar System (Hg from former name hydrargyrum, from Greek hydr- water and argyros silver) | 12 | 6 | 200.59(2) | 13.5336 | 234.43 | 629.88 | 0.14 | 2 | 0.085 |
| 81 | Tl | Thallium | the Greek 'thallos', meaning a green twig | 13 | 6 | 204.38(1)9 | 11.85 | 577 | 1746 | 0.129 | 1.62 | 0.85 |
| 82 | Pb | Lead | the Anglo-Saxon lead (plumbum in Latin)[6] | 14 | 6 | 207.2(1)2 4 | 11.342 | 600.61 | 2022 | 0.129 | 2.33 | 14 |
| 83 | Bi | Bismuth | the German 'Bisemutum' a corruption of 'Weisse Masse' meaning white mass | 15 | 6 | 208.98040(1)1 | 9.807 | 544.7 | 1837 | 0.122 | 2.02 | 0.009 |
| 84 | Po | Polonium | Poland, the native country of Marie Curie, who first isolated the element | 16 | 6 | [209]1 | 9.32 | 527 | 1235 | – | 2 | <0.001 |
| 85 | At | Astatine | the Greek 'astatos', meaning unstable | 17 | 6 | [210]1 | 7 | 575 | 610 | – | 2.2 | <0.001 |
| 86 | Rn | Radon | From radium, as it was first detected as an emission from radium during radioactive decay | 18 | 6 | [222]1 | 0.00973 | 202 | 211.3 | 0.094 | – | <0.001 |
| 87 | Fr | Francium | France | 1 | 7 | [223]1 | 1.87 | 300 | 950 | – | 0.7 | <0.001 |
| 88 | Ra | Radium | the Latin 'radius', meaning ray | 2 | 7 | [226]1 | 5.5 | 973 | 2010 | – | 0.9 | <0.001 |
| 89 | Ac | Actinium | the Greek 'actinos', meaning a ray | | 7 | [227]1 | 10.07 | 1323 | 3471 | 0.12 | 1.1 | <0.001 |
| 90 | Th | Thorium | Thor, the Scandinavian god of thunder | | 7 | 232.03806(2)1 2 | 11.72 | 2115 | 5061 | 0.113 | 1.3 | 9.6 |
| 91 | Pa | Protactinium | the Greek 'protos', meaning first, as a prefix to the element actinium, which is produced through the radioactive decay of protactinium | | 7 | 231.03588(2)1 | 15.37 | 1841 | 4300 | – | 1.5 | <0.001 |
| 92 | U | Uranium | Uranus, the seventh planet in the Solar System | | 7 | 238.02891(3)1 | 18.95 | 1405.3 | 4404 | 0.116 | 1.38 | 2.7 |
| 93 | Np | Neptunium | Neptune, the eighth planet in the Solar System | | 7 | [237]1 | 20.45 | 917 | 4273 | – | 1.36 | <0.001 |
| 94 | Pu | Plutonium | Pluto, a dwarf planet in the Solar System | | 7 | [244]1 | 19.84 | 912.5 | 3501 | – | 1.28 | <0.001 |
| 95 | Am | Americium | Americas, the continent where the element was first synthesized | | 7 | [243]1 | 13.69 | 1449 | 2880 | – | 1.3 | <0.001 |
| 96 | Cm | Curium | Pierre Curie, a physicist, and Marie Curie, a physicist and chemist | | 7 | [247]1 | 13.51 | 1613 | 3383 | – | 1.3 | <0.001 |
| 97 | Bk | Berkelium | Berkeley, California, USA, where the element was first synthesized | | 7 | [247]1 | 14.79 | 1259 | - | – | 1.3 | <0.001 |
| 98 | Cf | Californium | California, USA, where the element was first synthesized | | 7 | [251]1 | 15.1 | 1173 | - | – | 1.3 | <0.001 |
| 99 | Es | Einsteinium | Albert Einstein, physicist | | 7 | [252]1 | 13.5 | 1133 | – | – | 1.3 | 0 8 |
| 100 | Fm | Fermium | Enrico Fermi, physicist | | 7 | [257]1 | – | 1800 | – | – | 1.3 | 0 8 |
| 101 | Md | Mendelevium | Dmitri Mendeleyev, chemist and inventor | | 7 | [258]1 | – | 1100 | – | – | 1.3 | 0 8 |
| 102 | No | Nobelium | Alfred Nobel, chemist, engineer, innovator, and armaments manufacturer | | 7 | [259]1 | – | 1100 | – | – | 1.3 | 0 8 |
| 103 | Lr | Lawrencium | Ernest O. Lawrence, physicist | 3 | 7 | [262]1 | – | 1900 | – | – | 1.3 | 0 8 |
| 104 | Rf | Rutherfordium | Ernest Rutherford, chemist and physicist | 4 | 7 | [267]1 | – | – | – | – | – | 0 8 |
| 105 | Db | Dubnium | Dubna, Russia | 5 | 7 | [268]1 | – | – | – | – | – | 0 8 |
| 106 | Sg | Seaborgium | Glenn T. Seaborg, scientist | 6 | 7 | [269]1 | – | – | – | – | – | 0 8 |
| 107 | Bh | Bohrium | Niels Bohr, physicist | 7 | 7 | [270]1 | – | – | – | – | – | 0 8 |
| 108 | Hs | Hassium | Hesse, Germany, where the element was first synthesized | 8 | 7 | [269]1 | – | – | – | – | – | 0 8 |
| 109 | Mt | Meitnerium | Lise Meitner, physicist | 9 | 7 | [278]1 | – | – | – | – | – | 0 8 |
| 110 | Ds | Darmstadtium | Darmstadt, Germany, where the element was first synthesized | 10 | 7 | [281]1 | – | – | – | – | – | 0 8 |
| 111 | Rg | Roentgenium | Wilhelm Conrad Röntgen, physicist | 11 | 7 | [281]1 | – | – | – | – | – | 0 8 |
| 112 | Cn | Copernicium | Nicolaus Copernicus, astronomer | 12 | 7 | [285]1 | – | – | – | – | – | 0 8 |
| 113 | Nh | Nihonium[7] | Nihon, the main Japanese name for Japan; for research at the Riken (理研) institute. | 13 | 7 | [286]1 | – | – | – | – | – | 0 8 |
| 114 | Fl | Flerovium | Georgy Flyorov, physicist | 14 | 7 | [289]1 | – | – | – | – | – | 0 8 |
| 115 | Mc | Moscovium[7] | Named after Moscow, capital of Russia | 15 | 7 | [288]1 | – | – | – | – | – | 0 8 |
| 116 | Lv | Livermorium | Lawrence Livermore National Laboratory, in Livermore, California, U.S. | 16 | 7 | [293]1 | – | – | – | – | – | 0 8 |
| 117 | Ts | Tennessine[7] | US state Tennessee for work at Vanderbilt University and Oak Ridge National Laboratory there | 17 | 7 | [294]1 | – | – | – | – | – | 0 8 |
| 118 | Og | Oganesson[7] | Named after Yuri Oganesson, chemist involved in the synthesis of many elements | 18 | 7 | [294]1 | – | – | – | – | – | 0 8 |
| 9e99 | ~z | ~z | 9e99 | 9e99 | 9e99 | 9e99 | 9e99 | 9e99 | 9e99 | 9e99 | 9e99 |